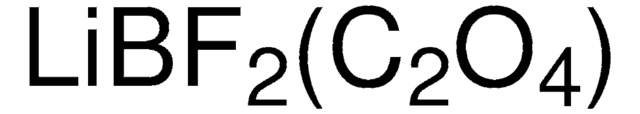推荐产品
等級
battery grade
品質等級
描述
Application: Battery manufacturing
化驗
99.9% trace metals basis
形狀
powder
環保替代產品特色
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
140 °C
負離子痕跡
chloride (Cl-): ≤5 ppm
sulfate (SO42-): ≤10 ppm
正離子痕跡
K: ≤10 ppm
Na: ≤5 ppm
應用
battery manufacturing
環保替代類別
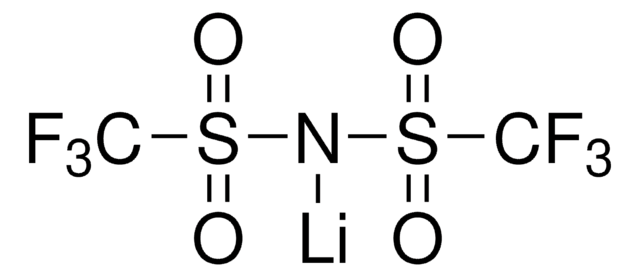
SMILES 字串
FS([N-]S(F)(=O)=O)(=O)=O.[Li+]
InChI
1S/F2NO4S2.Li/c1-8(4,5)3-9(2,6)7;/q-1;+1
InChI 密鑰
VDVLPSWVDYJFRW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Battery grade lithium bis(fluorosulfonyl)imide (LiFSI) is a white, powdery lithium salt often used as the source of lithium in high-performance electrolytes for lithium-ion batteries. LiFSI is soluble in water and many organics including the carbonates and ethers typically used in liquid electrolytes, like ethylene carbonate or dimethyl carbonate. Our battery grade LiFSI is differentiated by its high purity with low impurities of sodium, potassium, chloride, and sulfate, and low moisture content.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Green Chemistry. This product has been enhanced for energy efficiency. Click here for more information.
應用
Battery grade LiFSI is used as the source of lithium ions in battery electrolytes for LiBs. In comparison to LiPF6, LiFSI has marked advantages including a higher ionic conductivity in organic solvents and improved thermal stability. In addition, LiFSI has advantages in better stability against hydrolysis, lower aluminum corrosion with stability up to 4.7 V, higher transference number, and generally higher columbic efficiency for Li metal anode cycling.[3] Because of these advantages, many of the groundbreaking works to improve electrolytes use LiFSI. For example, researchers leveraged the improved solubility of LiFSI in ethers compared to LiTFSI or LiPF6 to formulate a LiFSI-based electrolyte that operates even at ultra-low temperatures like -30 °C, demonstrate cathodic stability up to 6 V vs Li/Li+, and achieve fast cycling with high columbic efficiency LiFSi is also commonly used as a co-salt with LiPF6 to improve the performance at high operating temperatures, for example 0.6 M LiFSI and 0.6 M LiPF6 in carbonate blends Researchers also often use LiFSI or a blend of LiFSI and LiTFSI as the source of lithium ions in polymer electrolytes, especially with Li metal anodes. LiFSI is shown to produce a LiF-rich solid-electrolyte interphase on Li metal surfaces, which promotes cycling with high coulombic efficiencies
相關產品
产品编号
说明
价格
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Muta. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门