911666
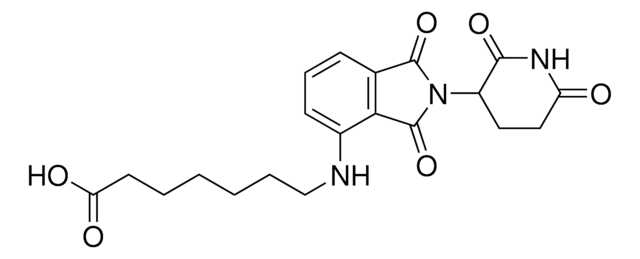
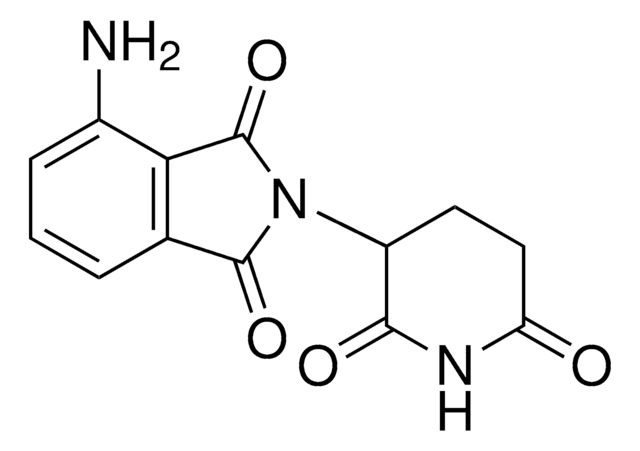
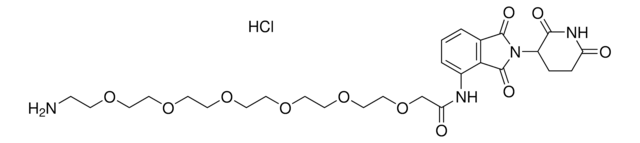
Pomalidomide-C6-NH2 hydrochloride
≥95%
别名:
4-((6-Aminohexyl)amino)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione hydrochloride, Crosslinker−E3 ligase ligand conjugate, Pomalidomide conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
推荐产品
ligand
pomalidomide
化驗
≥95%
形狀
powder
反應適用性
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
官能基
amine
儲存溫度
2-8°C
SMILES 字串
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NCCCCCCN)=O)NC1=O.Cl
InChI
1S/C19H24N4O4.ClH/c20-10-3-1-2-4-11-21-13-7-5-6-12-16(13)19(27)23(18(12)26)14-8-9-15(24)22-17(14)25;/h5-7,14,21H,1-4,8-11,20H2,(H,22,24,25);1H
InChI 密鑰
PBGMRXNTLPSDNR-UHFFFAOYSA-N
應用
其他說明
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Proteolysis Targeting Chimeras for the Selective Degradation of Mcl-1/Bcl-2 Derived from Nonselective Target Binding Ligands
Chemoselective Synthesis of Lenalidomide-Based PROTAC Library Using Alkylation Reaction
Identification of New Small-Molecule Inducers of Estrogen-related Receptor α (ERRα) Degradation
Discovery of MD-224 as a First-in-Class, Highly Potent and Efficacious PROTAC MDM2 Degrader Capable of Achieving Complete and Durable Tumor Regression
法律資訊
相關產品
訊號詞
Danger
危險聲明
危險分類
Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
商品
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门