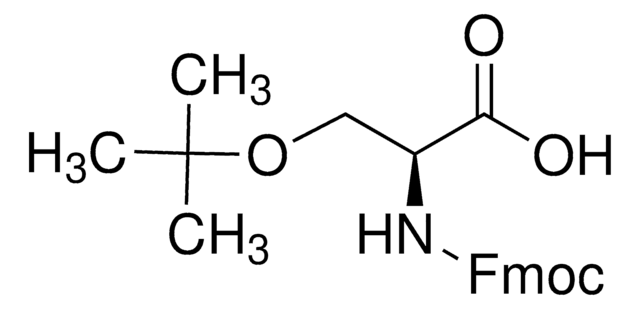
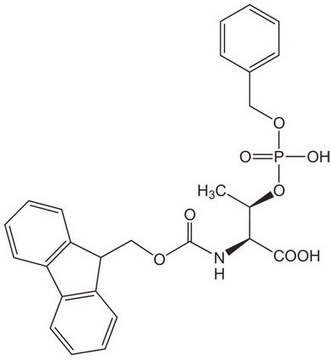
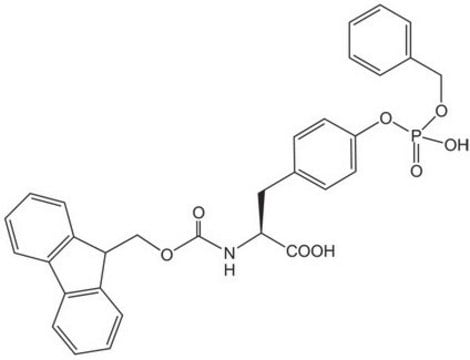
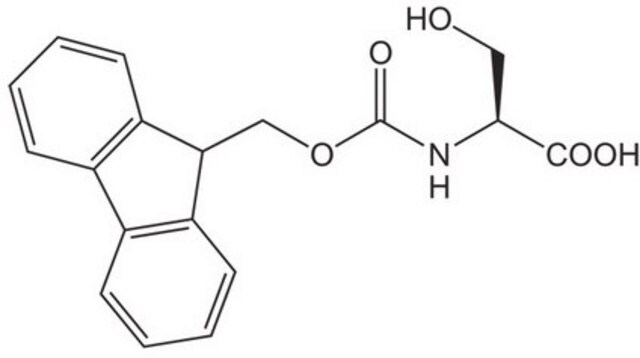
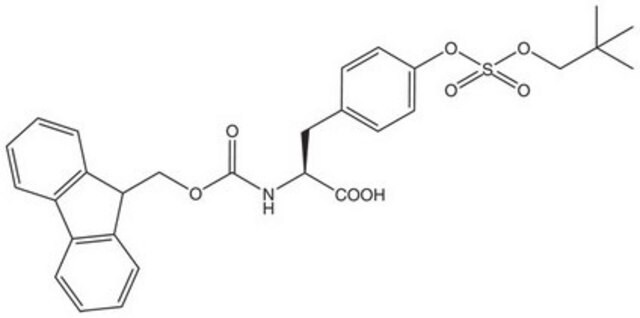
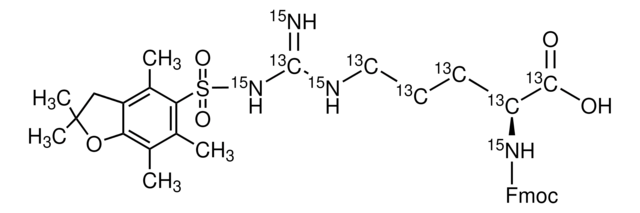
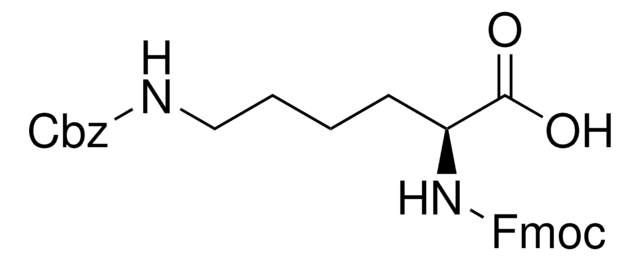
推荐产品
化驗
≥95%
形狀
solid
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
應用
peptide synthesis
官能基
Fmoc
儲存溫度
−20°C
應用
In the standard derivatives for introduction of phosphoserine and phosphothreonine in Fmoc SPPS, the phosphate side chain is monoprotected as a benzyl ester. The phosphate group therefore retains an acidic proton, which helps prevent beta-elimination of the phosphate group during the piperidine treatment utilized to remove the Fmoc protecting group in Fmoc SPPS. However, the acidic phosphate group can promote premature cleavage of peptides from hyperacid-labile resins such as 2-chlorotrityl resins. Furthermore, the phosphate forms piperidine salts during peptide assembly; these salts react with the activated Fmoc-amino acid during the coupling reaction, consuming valuable reagents and reducing their concentration.
Fmoc-Ser(PO(NPr)2)OH and Fmoc-Thr(PO(NPr)2)-OH are novel derivatives for introduction of phosphoserine and phosphothreonine in Fmoc SPPS, in which the phosphate group is fully protected as a phosphodiamidate. Model studies indicate these compounds, lacking the residual acidic proton, do not appear to suffer the aforementioned drawbacks of the currently employed monobenzylphosphate derivatives. Removal of the dimethyamino groups are removed during the course of the normal TFA cleavage and deprotection reaction.
Fmoc-Ser(PO(NPr)2)OH and Fmoc-Thr(PO(NPr)2)-OH are novel derivatives for introduction of phosphoserine and phosphothreonine in Fmoc SPPS, in which the phosphate group is fully protected as a phosphodiamidate. Model studies indicate these compounds, lacking the residual acidic proton, do not appear to suffer the aforementioned drawbacks of the currently employed monobenzylphosphate derivatives. Removal of the dimethyamino groups are removed during the course of the normal TFA cleavage and deprotection reaction.
法律資訊
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门