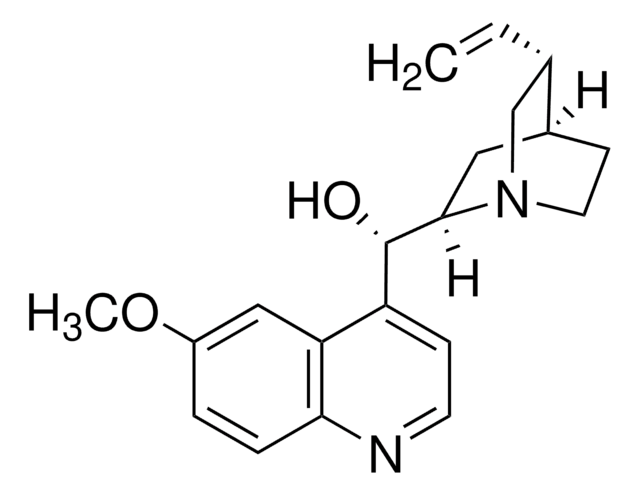
推荐产品
品質等級
化驗
85%
形狀
solid
光學活性
[α]23/D +228°, c = 0.5 in ethanol
mp
258-260 °C (lit.)
官能基
hydroxyl
SMILES 字串
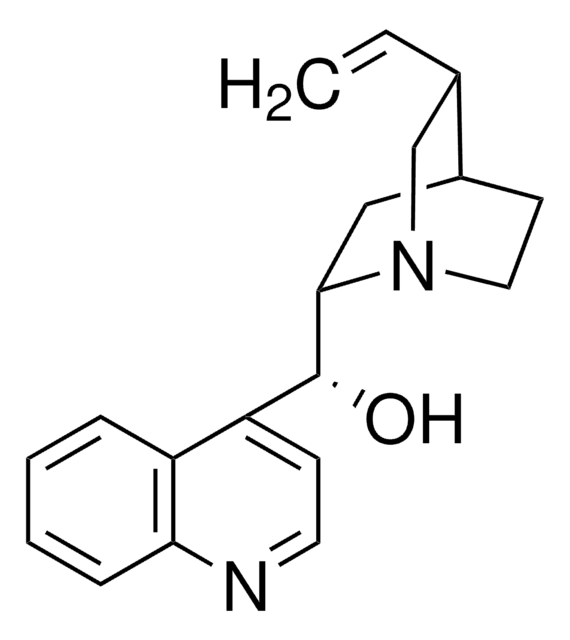
[H][C@@]12CCN(C[C@H]1C=C)[C@]([H])(C2)[C@@H](O)c3ccnc4ccccc34
InChI
1S/C19H22N2O/c1-2-13-12-21-10-8-14(13)11-18(21)19(22)16-7-9-20-17-6-4-3-5-15(16)17/h2-7,9,13-14,18-19,22H,1,8,10-12H2/t13-,14-,18+,19-/m0/s1
InChI 密鑰
KMPWYEUPVWOPIM-QAMTZSDWSA-N
一般說明
(+)-Cinchonine, one of the alkaloids found in the barks of cinchona tree, is mainly used in the treatment of malaria. It belongs to the monoclinic crystal system and P21 space group. The solubility of cinchonine can be improved by the formation of inclusion complexes with cyclodextrins.
應用
(+)-Cinchonine, in the presence of lithium diisopropylamide (LDA) forms a complex, which can catalyze the asymmetric conjugate addition of benzyl- and alkylphosphonates to aromatic and heteroaromatic nitroalkenes to form the corresponding adducts.
其他說明
残留二氢金鸡宁
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Skin Sens. 1A
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Cinchonine catalyzed diastereo-and enantioselective Michael addition of a-lithiated phosphonates to nitroalkenes.
Rai V, et al.
Tetrahedron Asymmetry, 18(22), 2719-2726 (2007)
Song CE.
Cinchona Alkaloids in Synthesis and Catalysis: Ligands, Immobilization and Organocatalysis, 2-3 (2009)
The molecular and crystal structure of the alkaloid cinchonine.
Oleksyn B, et al.
Acta Crystallographica Section B, Structural Science, 35(2), 440-444 (1979)
Qing Gu et al.
Organic letters, 13(19), 5192-5195 (2011-09-15)
Desymmetrization of cyclohexadienones bearing a bisphenylsulfonyl methylene group via asymmetric Michael reaction catalyzed by cinchonine-derived urea was realized to afford a series of highly enantioenriched polycyclic cyclohexenones in high yields and ee's.
Yu-Hua Liao et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(21), 6679-6687 (2012-04-14)
An asymmetric conjugate addition of 3-monosubstituted oxindoles to a range of (E)-1,4-diaryl-2-buten-1,4-diones, catalyzed by commercially available cinchonine, is described. This organocatalytic asymmetric reaction affords a broad range of 3,3'-disubstituted oxindoles that contain a 1,4-dicarbonyl moiety and vicinal quaternary and tertiary
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门