推荐产品
品質等級
化驗
≥95% (sulfur, elemental analysis)
形狀
powder
成份
active SO2, ~50%
carbon, 28.3-31.6%
儲存溫度
2-8°C
SMILES 字串
[O-]S(=O)[N+]12CC[N+](CC1)(CC2)S([O-])=O
InChI
1S/C6H12N2O4S2/c9-13(10)7-1-2-8(5-3-7,6-4-7)14(11)12/h1-6H2
InChI 密鑰
RWISEVUOFYXWFO-UHFFFAOYSA-N
應用
1,4-二氮杂双环[2.2.2]辛烷双(二氧化硫)加合物(DABSO)是以一种电荷转移络合物,可用作以下应用的二氧化硫替代物的:
也可用于活化DMSO和邻乙烯基苯胺,分别用于合成N-芳基-1H-苯并[d]咪唑-1-胺和4-芳基喹啉。
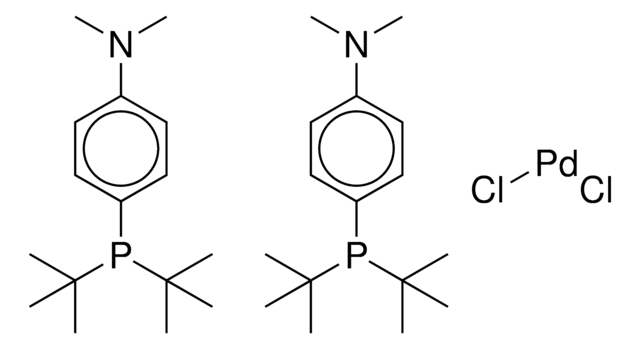
- 钯催化的氨基磺酰化工艺。
- 与芳基溴化物反应合成芳基钠。
也可用于活化DMSO和邻乙烯基苯胺,分别用于合成N-芳基-1H-苯并[d]咪唑-1-胺和4-芳基喹啉。
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
4.1B - Flammable solid hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Bao Nguyen et al.
Journal of the American Chemical Society, 132(46), 16372-16373 (2010-10-30)
The palladium-catalyzed three-component coupling of aryl iodides, sulfur dioxide, and hydrazines to deliver aryl N-aminosulfonamides is described. The colorless crystalline solid DABCO·(SO(2))(2) was used as a convenient source of sulfur dioxide. The reaction tolerates significant variation of both the aryl
Synthesis of sodium aryl sulfinates from aryl bromides employing 1, 4-diazabicyclo [2.2. 2] octane bis (sulfur dioxide) adduct (DABSO) as a bench-stable, gas-free alternative to SO2.
Skillinghaug B, et al.
Tetrahedron Letters, 57(5), 533-536 (2016)
Carbon annulation of ortho-vinylanilines with dimethyl sulfoxide to access 4-aryl quinolines.
Yuan J, et al.
Organic & Biomolecular Chemistry, 15(6), 1334-1337 (2017)
Palladium-catalyzed aminosulfonylation of aryl halides.
Nguyen B, et al.
Journal of the American Chemical Society, 132(46), 16372-16373 (2010)
Palladium-catalyzed annulation of 2-(aryldiazenyl) aniline with dimethyl sulfoxide to access N-aryl-1H-benzo [d] imidazol-1-amine.
Wang H, et al.
Tetrahedron Letters, 58(40), 3875-3878 (2017)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![1,4-二叠氮双环[2.2.2]辛烷 ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)
![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)