About This Item
推荐产品
化驗
95%
反應適用性
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-C Bond Formation
reagent type: ligand
reaction type: Cross Couplings
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
官能基
phosphine
SMILES 字串
CCCCP([C@]12C[C@H]3C[C@H](C[C@H](C3)C1)C2)[C@@]45C[C@@H]6C[C@@H](C[C@@H](C6)C4)C5
InChI
1S/C24H39P/c1-2-3-4-25(23-11-17-5-18(12-23)7-19(6-17)13-23)24-14-20-8-21(15-24)10-22(9-20)16-24/h17-22H,2-16H2,1H3/t17-,18+,19-,20-,21+,22-,23-,24-
InChI 密鑰
HTJWUNNIRKDDIV-FECFBMJZSA-N
一般說明
配套销售
cataCXium® A通常用作交叉偶联反应的催化剂,如Suzuki和Sonogashira反应。
應用
其他应用:
- 钯催化的芳基和杂芳基卤化物的羰基化
- 钯催化的(杂)芳腈的合成
- 钯催化的芳基卤化物的氨基羰基化
特點和優勢
- 反应条件温和,
- 催化剂负载量低,
- 产率高,转化率高
法律資訊
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
商品
cataCXium® - Ligands and Complexes for Efficient Cross-Coupling Reactions. Cross-coupling reactions are an important class of catalytic transformations with applications in polymer science as well as in the fine chemicals and pharmaceutical industries.
cataCXium® - Ligands and Complexes for Efficient Cross-Coupling Reactions. Cross-coupling reactions are an important class of catalytic transformations with applications in polymer science as well as in the fine chemicals and pharmaceutical industries.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![[(二(1-金刚烷基)丁基膦基)-2-(2′-氨基-1,1′-联苯基)]钯(II)甲磺酸酯 95%](/deepweb/assets/sigmaaldrich/product/structures/391/492/af15708b-9501-44ae-a25f-d3574589a865/640/af15708b-9501-44ae-a25f-d3574589a865.png)



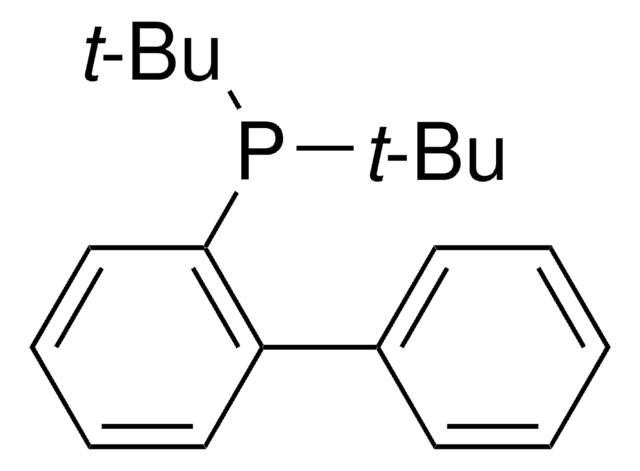

![反式二-(M)-双[2-(二邻甲苯基膦)苄基]乙酸二钯(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/291/406/8cdb9bda-d2bf-41c5-adfb-c3cc57cf62e8/640/8cdb9bda-d2bf-41c5-adfb-c3cc57cf62e8.png)
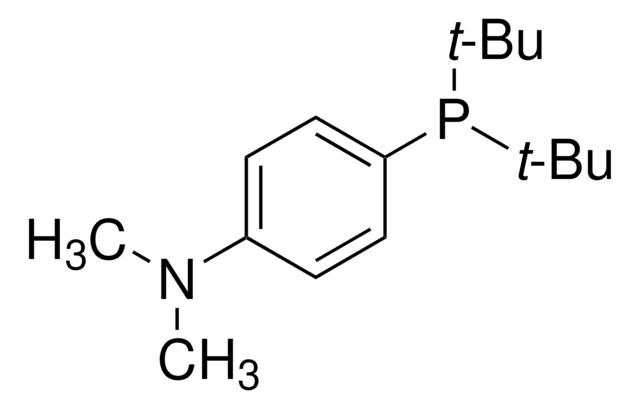
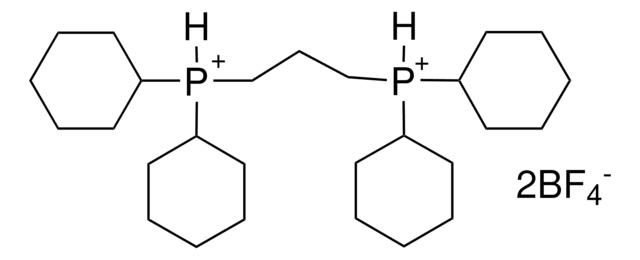
![1,4-二氮杂二环[2.2.2]辛烷二(二氧化硫)加合物 ≥95% (sulfur, elemental analysis)](/deepweb/assets/sigmaaldrich/product/structures/158/739/a9df497b-883d-40f1-ac45-bf699dcee9f9/640/a9df497b-883d-40f1-ac45-bf699dcee9f9.png)