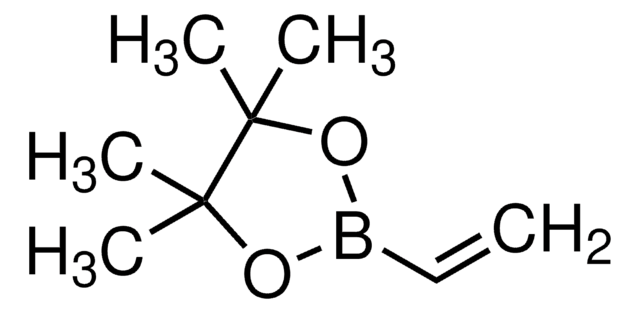
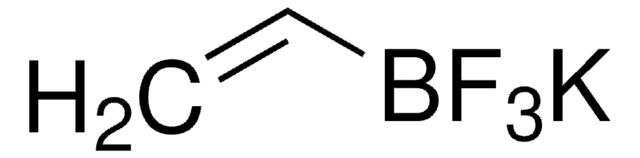
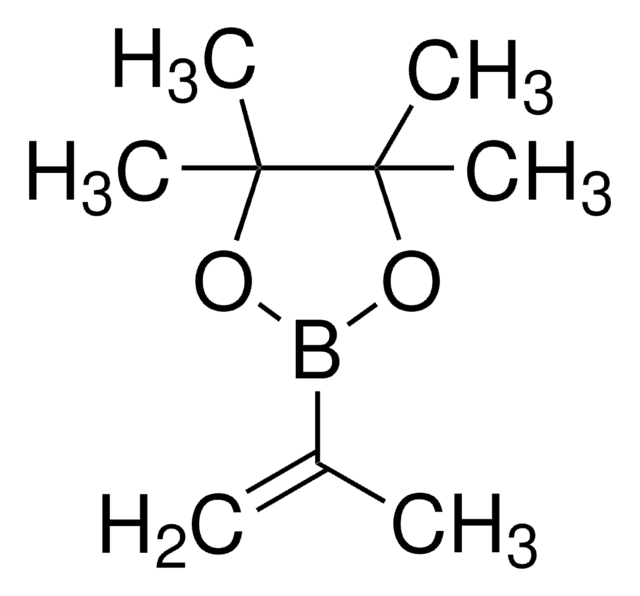
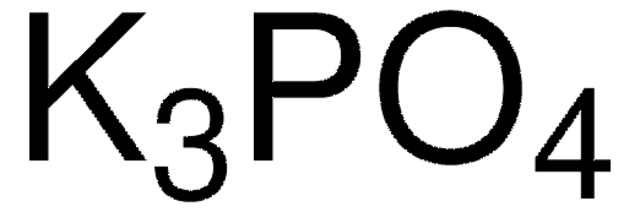推荐产品
品質等級
化驗
95%
形狀
liquid
折射率
n20/D 1.447
密度
0.935 g/mL at 25 °C
官能基
ether
儲存溫度
2-8°C
SMILES 字串
CCO\C=C\B1OC(C)(C)C(C)(C)O1
InChI
1S/C10H19BO3/c1-6-12-8-7-11-13-9(2,3)10(4,5)14-11/h7-8H,6H2,1-5H3/b8-7+
InChI 密鑰
MRAYNLYCQPAZJN-BQYQJAHWSA-N
應用
trans-2-Ethoxyvinylboronic acid pinacol ester is a boronic ester commonly used in Suzuki-Miyaura cross-coupling.
This reaction is a key step to synthesize:
This reaction is a key step to synthesize:
- Azaindole and diazaindoles from chloroamino-N-heterocycles.
- Doryanine and its derivatives from 2-bromobenzoic acid.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
188.1 °F
閃點(°C)
86.7 °C
Structure-and reactivity-based development of covalent inhibitors of the activating and gatekeeper mutant forms of the epidermal growth factor receptor (EGFR).
Ward, Richard A et al.
Journal of Medicinal Chemistry, 56(17), 7025-7048 (2013)
Total Synthesis of the Illicium-Derived Sesquineolignan Simonsol C.
Nugent, Jeremy et al.
Organic Letters, 18(15), 3798-3801 (2016)
Two-step synthesis of aza-and diazaindoles from chloroamino-N-heterocycles using ethoxyvinylborolane.
Whelligan, Daniel K et al.
The Journal of Organic Chemistry, 75(1), 11-15 (2009)
Efficient and rapid synthesis of N-substituted isoquinolin-1-ones under mild conditions: Facile access to doryanine derivatives
Takwale AD, et al.
Tetrahedron Letters, 60(18), 1259-1261 (2019)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)