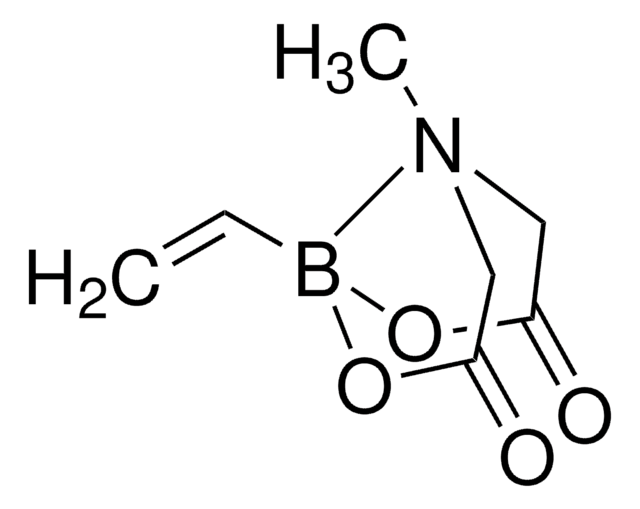
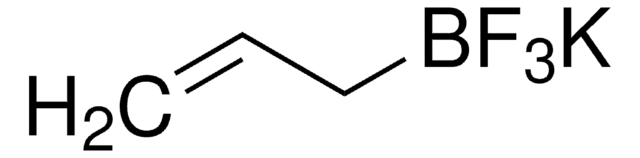
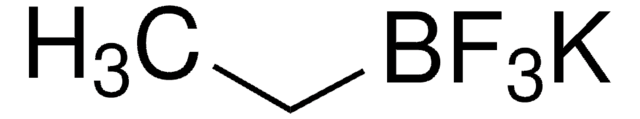
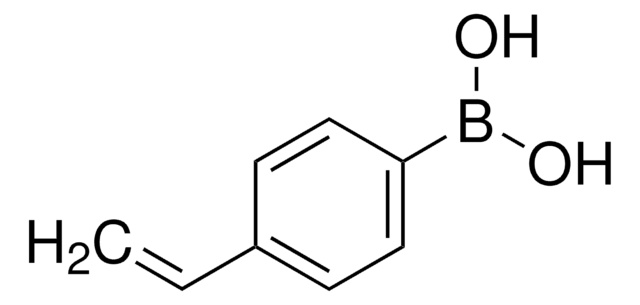
推荐产品
化驗
95%
形狀
solid
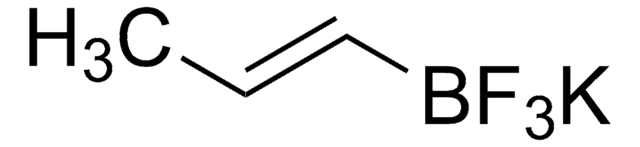
SMILES 字串
[K+].F[B-](F)(F)C=C
InChI
1S/C2H3BF3.K/c1-2-3(4,5)6;/h2H,1H2;/q-1;+1
InChI 密鑰
ZCUMGICZWDOJEM-UHFFFAOYSA-N
一般說明
乙烯基三氟硼酸钾是一种对空气和水稳定的有机三氟硼酸钾,可在相对温和的条件下用于偶联反应。
應用
- Suzuki Miyaura 交叉偶联反应和聚合反应
- 光子晶体的合成
- 用于染料敏化太阳能电池的敏化剂的合成
- 曼尼希/非对映选择性加氢胺化反应顺序
有机三氟硼酸盐作为多功能和稳定的硼酸替代物。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
商品
Potassium trifluoroborates are a special class of organoboron reagents that offer several advantages over the corresponding boronic acids and esters in that they are moisture- and air-stable, and are remarkably compliant with strong oxidative conditions.
Potassium trifluoroborates are a special class of organoboron reagents that offer several advantages over the corresponding boronic acids and esters in that they are moisture- and air-stable, and are remarkably compliant with strong oxidative conditions.
These bench stable Potassium Organotrifluoroborates are useful for Suzuki-Miyaura cross-coupling reactions and have also been used for a variety of other C-C bond forming reactions. Importantly, these reagents are compatible with a wide range of functional groups and are stable to many commonly used and harsh reaction conditions.
These bench stable Potassium Organotrifluoroborates are useful for Suzuki-Miyaura cross-coupling reactions and have also been used for a variety of other C-C bond forming reactions. Importantly, these reagents are compatible with a wide range of functional groups and are stable to many commonly used and harsh reaction conditions.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)