所有图片(3)
About This Item
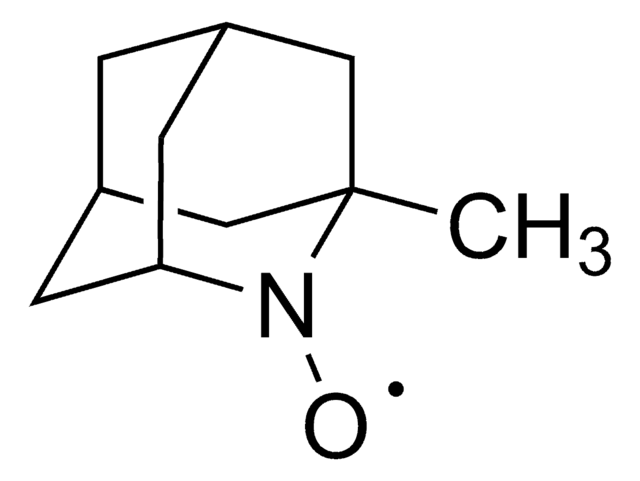
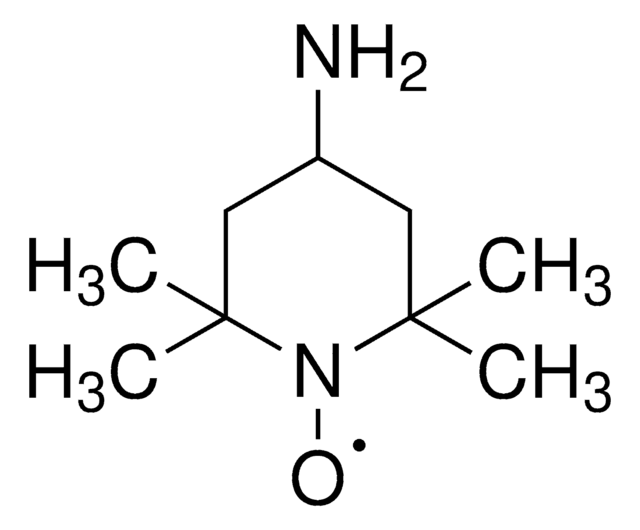
经验公式(希尔记法):
C9H14NO
CAS号:
分子量:
152.21
MDL號碼:
分類程式碼代碼:
12352000
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
90%
形狀
powder
反應適用性
reagent type: oxidant
mp
182-189 °C (D)
儲存溫度
2-8°C
SMILES 字串
[O]N1[C@@H]2C[C@H]3C[C@@H](C2)C[C@@H]1C3
InChI
1S/C9H14NO/c11-10-8-2-6-1-7(4-8)5-9(10)3-6/h6-9H,1-5H2/t6-,7+,8-,9+
InChI 密鑰
BCJCJALHNXSXKE-SPJNRGJMSA-N
一般說明
2-氮杂金刚烷- N -氧代 (AZADO) 是一种稳定的氮氧自由基,被广泛地应用于催化醇类的氧化反应。
應用
2-氮杂金刚烷- N -氧代 (AZADO) 可用于以下研究:
- 用作木材纤维素氧化的催化剂。
- 雅库酰胺 A(一种从海绵 Ceratopsion sp. 中提取的潜在细胞毒素)全合成的催化剂。
- 作为 ( S )-缩水甘油氧化的氧化剂。
其他客户在看
Masatoshi Shibuya et al.
Journal of the American Chemical Society, 128(26), 8412-8413 (2006-06-29)
Development of a stable nitroxyl radical class of catalysts, 2-azaadamantane N-oxyl (AZADO) and 1-Me-AZADO, for highly efficient oxidation of alcohols is described. AZADO and 1-Me-AZADO exhibit superior catalytic proficiency to TEMPO, converting various sterically hindered alcohols to the corresponding carbonyl
Takefumi Kuranaga et al.
Journal of the American Chemical Society, 135(14), 5467-5474 (2013-03-19)
Here we report the first total synthesis and the complete stereochemical assignment of yaku'amide A. Yaku'amide A (1) was isolated from a sponge Ceratopsion sp. as an extremely potent cytotoxin. Its structure was determined except for the C4-stereochemistry in the
Ming Zhang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(10), 3937-3941 (2015-01-22)
An increased supply of scarce or inaccessible natural products is essential for the development of more sophisticated pharmaceutical agents and biological tools, and thus the development of atom-economical, step-economical and scalable processes to access these natural products is in high
Takuya Isogai et al.
Biomacromolecules, 11(6), 1593-1599 (2010-05-18)
Curdlan, amylodextrin, and regenerated cellulose fiber were subjected to electromediated oxidation with a 4-acetamido-TEMPO catalyst in a buffer at pH 6.8 without NaClO or NaClO(2). More than 90% of the C6 primary hydroxyls of Curdlan and amylodextrin were converted to
商品
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![9-氮杂双环[3.3.1]壬烷N-氧基 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)