推荐产品
折射率
n20/D 1.4353
品質等級
密度
0.842 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
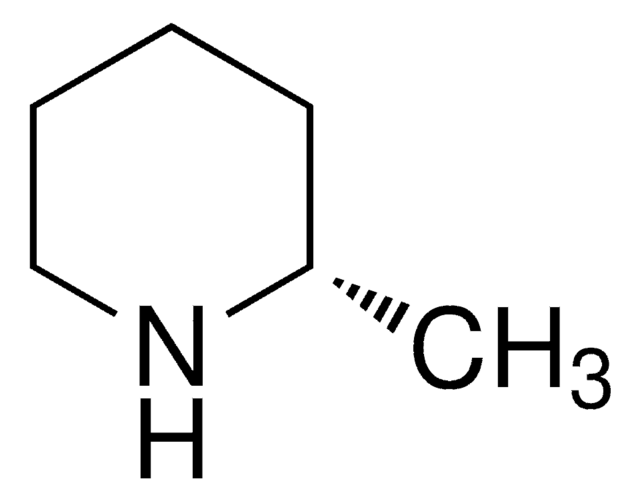
SMILES 字串
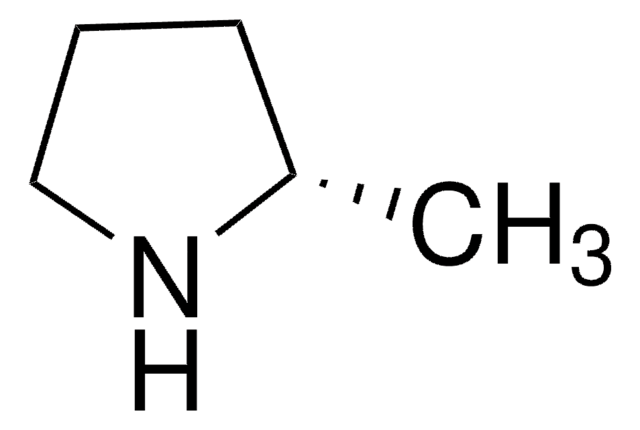
C[C@@H]1CCCN1
InChI
1S/C5H11N/c1-5-3-2-4-6-5/h5-6H,2-4H2,1H3/t5-/m1/s1
InChI 密鑰
RGHPCLZJAFCTIK-RXMQYKEDSA-N
正在寻找类似产品? 访问 产品对比指南
應用
(R)-(−)-2-Methylpyrrolidine, an optically active amine that can be used as a key building block to synthesize:
- 4,5-fused pyridazinone derivatives are applicable as potent histamine H3 receptor antagonists.
- Naphthalenoid histamine H3 receptor antagonist.
- Pyrrolo[2,3-d]pyrimidine derivatives as potent inhibitors of leucine-rich repeat kinase 2.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
45.0 °F - closed cup
閃點(°C)
7.22 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Synthesis and structure?activity relationships of 4, 5-fused pyridazinones as histamine H 3 receptor antagonists
Tao, Ming, et al.
Bioorganic & Medicinal Chemistry Letters, 21.20, 6126-6130 (2011)
Identification of pyridazin-3-one derivatives as potent, selective histamine H 3 receptor inverse agonists with robust wake activity
Hudkins, Robert L., et al.
Bioorganic & Medicinal Chemistry Letters, 21.18, 5493-5497 (2011)
Synthesis and evaluation of pyridazinone?phenethylamine derivatives as selective and orally bioavailable histamine H 3 receptor antagonists with robust wake-promoting activity
reddy Dandu, Reddeppa, et al.
Bioorganic & Medicinal Chemistry Letters, 21.21, 6362-6365 (2011)
Organic Process Research & Development, 11, 1004-1004 (2007)
A new class of potent non-imidazole H 3 antagonists: 2-aminoethylbenzofurans
Cowart, Marlon, et al.
Bioorganic & Medicinal Chemistry Letters, 14.3, 689-693 (2004)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持