所有图片(1)
About This Item
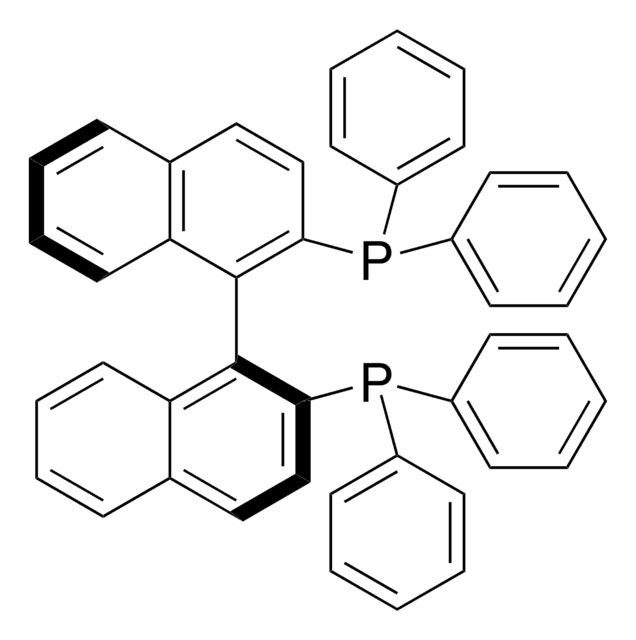
经验公式(希尔记法):
C56H42O2Si2
CAS号:
分子量:
803.10
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
96%
形狀
solid
光學活性
[α]20/D +114°, c = 1 in chloroform
mp
103-145 °C
SMILES 字串
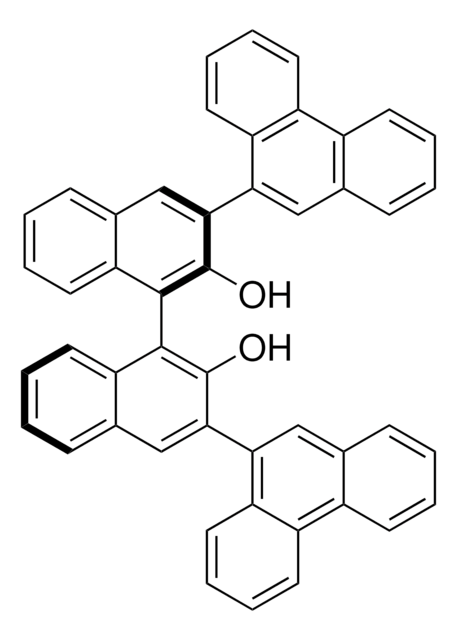
Oc1c(cc2ccccc2c1-c3c(O)c(cc4ccccc34)[Si](c5ccccc5)(c6ccccc6)c7ccccc7)[Si](c8ccccc8)(c9ccccc9)c%10ccccc%10
InChI
1S/C56H42O2Si2/c57-55-51(59(43-25-7-1-8-26-43,44-27-9-2-10-28-44)45-29-11-3-12-30-45)39-41-23-19-21-37-49(41)53(55)54-50-38-22-20-24-42(50)40-52(56(54)58)60(46-31-13-4-14-32-46,47-33-15-5-16-34-47)48-35-17-6-18-36-48/h1-40,57-58H
InChI 密鑰
STBZSRVMGWTCOU-UHFFFAOYSA-N
應用
手性布朗斯特酸 (674745) 的前体,用于催化不对称氮杂 Diels-Alder 反应,生成双环内酰胺。 该手性联萘酚的稀土金属络合物可催化氨基烯烃的分子内氢胺化反应,生成手性吡咯烷。
(R)-3,3′-Bis(triphenylsilyl)-1,1′-bi-2-naphthol reacts with trimethylaluminum to form a chiral organoaluminum reagent, which can catalyze the asymmetric Diels-Alder reaction of cyclopentadiene and methyl acrylate to form the corresponding Diels-Alder adduct.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Asymmetric Diels-Alder Reaction of Cyclopentadiene and Methyl Acrylate Catalyzed by Chiral Organoaluminum Reagents.
Bulletin of the Chemical Society of Japan, 65(12), 3501-3503 (1992)
Hua Liu et al.
Organic letters, 8(26), 6023-6026 (2006-12-15)
[Structure: see text] The first chiral Brønsted acid-catalyzed asymmetric direct aza hetero-Diels-Alder reaction has been described. The phosphoric acids, prepared from binol and H8-binol derivatives, have shown catalytic ability for the reaction of cyclohexenone with N-PMP-benzaldimine. A chiral phosphoric acid
Denis V Gribkov et al.
Journal of the American Chemical Society, 128(11), 3748-3759 (2006-03-16)
Chiral 3,3'-bis(trisarylsilyl)-substituted binaphtholate rare earth metal complexes (R)-[Ln{Binol-SiAr3}(o-C6H4CH2NMe2)(Me2NCH2Ph)] (Ln = Sc, Lu, Y; Binol-SiAr3 = 3,3'-bis(trisarylsilyl)-2,2'-dihydroxy-1,1'-binaphthyl; Ar = Ph (2-Ln), 3,5-xylyl (3-Ln)) and (R)-[La{Binol-Si(3,5-xylyl)3}{E(SiMe3)2}(THF)2] (E = CH (4a), N (4b)) are accessible via facile arene, alkane, and amine elimination. They
商品
We present an article concerning BINOL and Derivatives.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持