推荐产品
品質等級
化驗
98%
反應適用性
reagent type: cross-linking reagent
mp
70-73 °C (lit.)
官能基
NHS ester
imide
maleimide
儲存溫度
−20°C
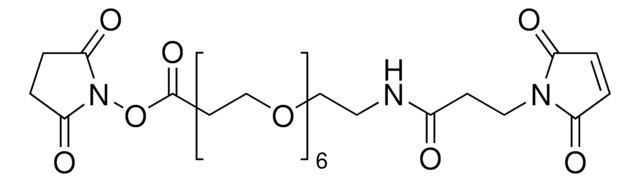
SMILES 字串
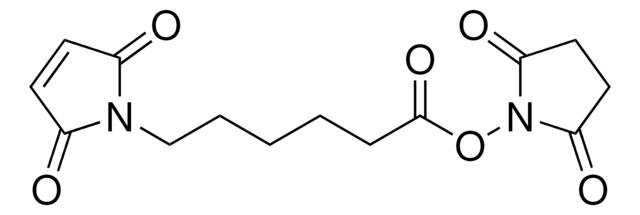
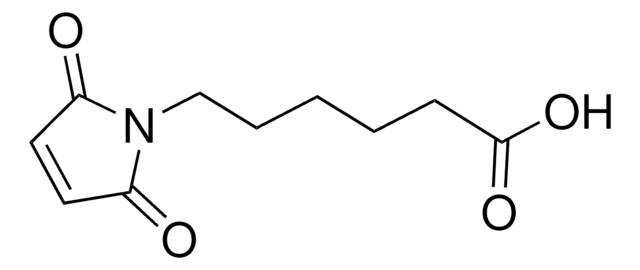
O=C(ON(C(CC1)=O)C1=O)CCCCCN2C(C=CC2=O)=O
InChI
1S/C14H16N2O6/c17-10-5-6-11(18)15(10)9-3-1-2-4-14(21)22-16-12(19)7-8-13(16)20/h5-6H,1-4,7-9H2
InChI 密鑰
VLARLSIGSPVYHX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
6-马来酰亚胺己酸N-羟基琥珀酰亚胺酯可用于:
- 通过马来酰亚胺硫醇连接反应合成马来酰亚胺活化碳水化合物,用于含半胱氨酸的多肽和蛋白质的位点特异性糖基化。
- 含有马来酰亚胺侧链作为抗肿瘤剂的阿霉素葡糖苷酸前药的合成。
- 将寡核苷酸与底物上的氨基交联以制备DNA微阵列。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Synthesis and antitumor efficacy of a β-glucuronidase-responsive albumin-binding prodrug of doxorubicin.
Legigan T, et al.
Journal of Medicinal Chemistry, 55(9), 4516-4520 (2012)
Microarray fabrication with covalent attachment of DNA using bubble jet technology.
Okamoto T, et al.
Nature Biotechnology, 18(4), 438-438 (2000)
Synthesis of maleimide-activated carbohydrates as chemoselective tags for site-specific glycosylation of peptides and proteins.
Ni J, et al.
Bioconjugate Chemistry, 14(1), 232-238 (2003)
Y Nakano et al.
International archives of allergy and immunology, 120(3), 199-208 (1999-12-11)
We have previously reported that ovalbumin (OVA) coupled with liposome via glutaraldehyde (GA) induced OVA-specific- and IgE-selective unresponsiveness in mice. In this study, OVA-liposome conjugates were made using four different coupling protocols: via GA, N-(6-maleimidocaproyloxy) succinimide (EMCS), disuccinimidyl suberate (DSS)
N J Maeji et al.
Journal of immunological methods, 146(1), 83-90 (1992-01-21)
Recently, the multipin approach for simultaneous multiple peptide synthesis was applied to the analysis of T cell determinants by using a novel cleavage method (Maeji et al., 1990). A diketopiperazine forming linker allowed cleavage of peptides into aqueous buffer which
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门