所有图片(2)
About This Item
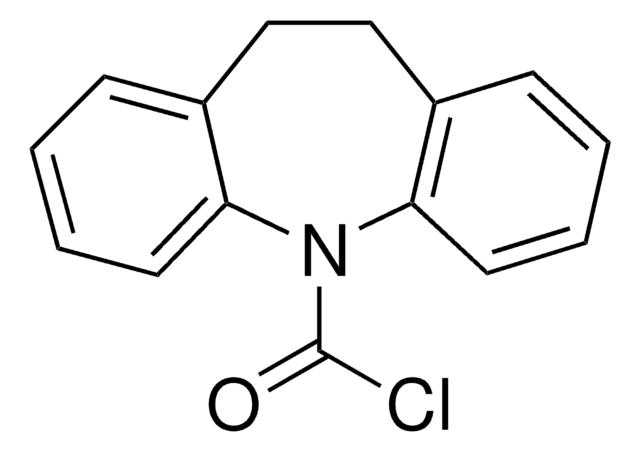
经验公式(希尔记法):
C15H10ClNO
CAS号:
分子量:
255.70
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
90%
mp
149-153 °C (lit.)
官能基
chloro
SMILES 字串
ClC(=O)N1c2ccccc2C=Cc3ccccc13
InChI
1S/C15H10ClNO/c16-15(18)17-13-7-3-1-5-11(13)9-10-12-6-2-4-8-14(12)17/h1-10H
InChI 密鑰
APJYHXJGXDPGBA-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Dibenz [b,f]azepine-5-carbonyl chloride or 5H-dibenz [b,f]azepine-5-carbonyl chloride is a tricyclic heterocyclic compound that can be synthesized from 5H-dibenz[ b,f]azepine.
應用
Dibenz [b,f]azepine-5-carbonyl chloride may be used in the preparation of trans-10,11-dibromo-10,11-dihydro-5H-dibenz[b,f]azepine-5-carbonyl chloride via bromination using bromine. It may also be used to prepare urea derivatives, which are potent P2X4 receptor (purinergic receptor) antagonists.
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Maoqun Tian et al.
Bioorganic & medicinal chemistry, 22(3), 1077-1088 (2014-01-15)
Antagonists for the P2 receptor subtype P2X4, an ATP-activated cation channel receptor, have potential as novel drugs for the treatment of neuropathic pain and other inflammatory diseases. In the present study, a series of 47 carbamazepine derivatives including 32 novel
Kinetic evidence for rate determination during the nucleophilic step of olefin bromination. The case of 5H-dibenz [b, f] azepine-5-carbonyl chloride.
Bellucci G and Chiappe C.
The Journal of Organic Chemistry, 58(25), 7120-7127 (1993)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门