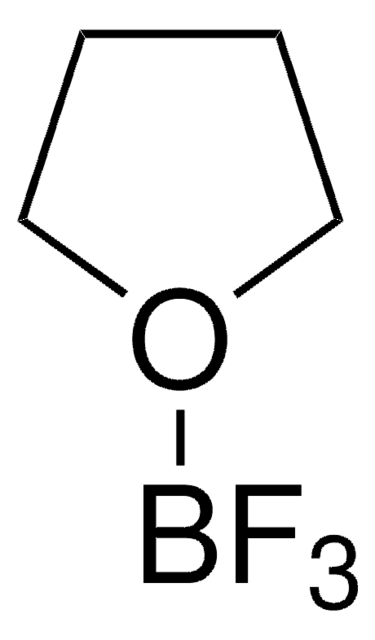
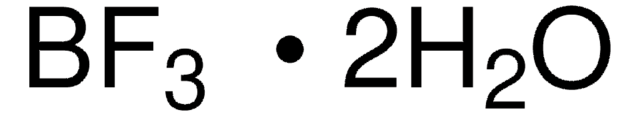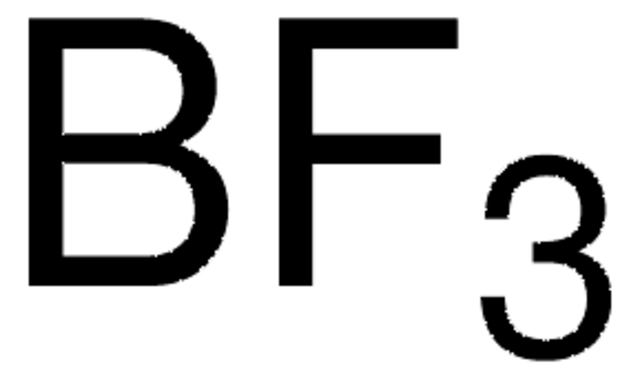推荐产品
等級
electronic grade
蒸汽密度
2.38 (21 °C, vs air)
化驗
≥99.99%
形狀
gas
反應適用性
core: boron
reagent type: catalyst
雜質
<10 ppm Carbon dioxide (CO2)
<10 ppm Nitrogen(N2) + oxygen (O2)
<10 ppm Other Sulfates
<10 ppm Sulfur dioxide (SO2)
<50 ppm Silicon tetrafluoride (SiF4)
bp
−100 °C (lit.)
mp
−127 °C (lit.)
轉變溫度
critical temperature −12.3 °C
SMILES 字串
FB(F)F
InChI
1S/BF3/c2-1(3)4
InChI 密鑰
WTEOIRVLGSZEPR-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
基础材料的原子序数:5 Boron
應用
最近应用于路易斯酸活化晶界增强CaF2 电导率的研究。
推薦產品
建议使用Monel控制阀Z261793。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 2 Inhalation - Eye Dam. 1 - Press. Gas Compr. Gas - Skin Corr. 1A
安全危害
儲存類別代碼
2A - Gases
水污染物質分類(WGK)
WGK 1
個人防護裝備
Faceshields, Gloves, Goggles, multi-purpose combination respirator cartridge (US)
其他客户在看
Solid State Ionics, 86-88, 581-581 (1996)
T Mizuno et al.
The Journal of chemical physics, 136(7), 074305-074305 (2012-03-01)
Recoil frame photoelectron angular distributions (RFPADs) of BF(3) molecules are presented over the energy region of the shape resonance in the F 1s continuum. Time-dependent density functional theory calculations are also given to understand the shape resonance dynamics. The RFPADs
The influence of the acidity of ionic liquids on catalysis.
Xinjiang Cui et al.
ChemSusChem, 3(9), 1043-1047 (2010-08-18)
Ramanathan Rajaganesh et al.
Carbohydrate research, 345(12), 1649-1657 (2010-06-29)
BF(3).Et(2)O-catalysed O-glycosylation of 1,2,3-tri-O-acetyl-4,6-O-butylidene- and ethylidene-beta-d-glucopyranose with different aliphatic and aromatic alcohols proceeds for the most part with complete retention of anomeric configuration. Antioxidant activity of O-glycosides shows significant inhibition (IC(50) approximately 77%). 1,3-Dipolar cycloaddition of terminal alkyne derivatives of
G K Surya Prakash et al.
The Journal of organic chemistry, 74(22), 8659-8668 (2009-10-30)
BF(3)-monohydrate is found to be an efficient and strong acid catalyst as well as an effective protosolvating medium suitable for the hydroxyalkylation of arenes with aromatic aldehydes. This reaction has been extended to aromatic dialdehydes, such as terephthalic dicarboxaldehyde and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门