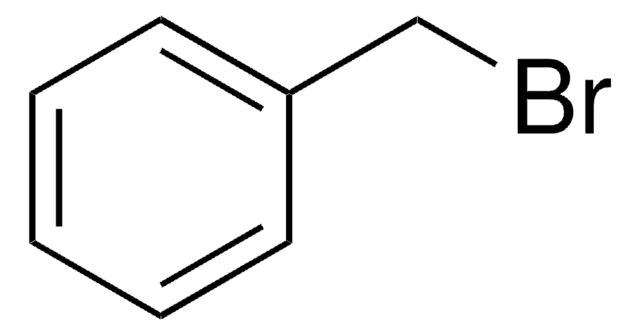
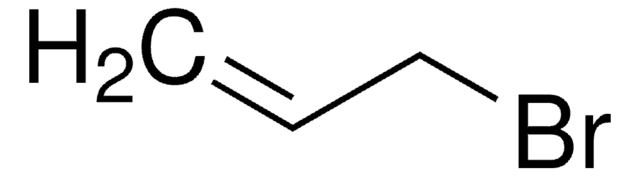
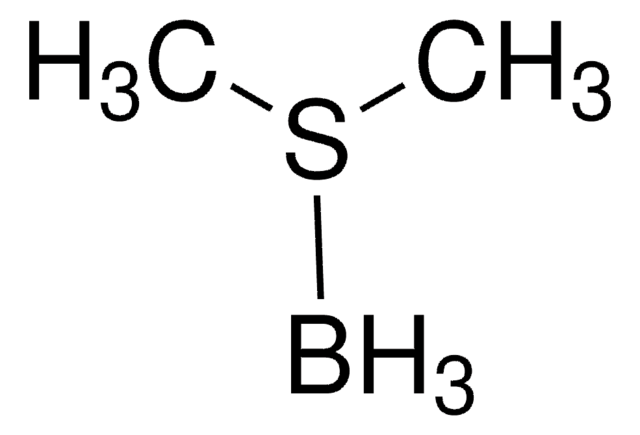
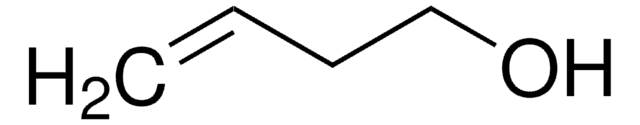
推荐产品
反應適用性
reagent type: reductant
濃度
0.4 M in hexanes
bp
68-70 °C
密度
0.691 g/mL at 25 °C
SMILES 字串
B1C2CCCC1CCC2
InChI
1S/C8H15B/c1-3-7-5-2-6-8(4-1)9-7/h7-9H,1-6H2/t7-,8+
InChI 密鑰
FEJUGLKDZJDVFY-OCAPTIKFSA-N
正在寻找类似产品? 访问 产品对比指南
應用
烯烃保护基团†
反应物用于:
反应物用于:
- 线性SPPS合成泛素衍生物
- 铜催化有机硼化合物与伯烷基卤化物和拟卤化物发生交叉偶联反应
- 将烯烃分子内插入钯-氮键
- 制备(膦酰基乙酰基)鸟氨酸以研究对酵母中精氨酸生物合成基因的影响
- Hetero-Diels-Alder反应合成螺环生物碱
訊號詞
Danger
危險分類
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 Inhalation - STOT SE 3 - Water-react 1
標靶器官
Central nervous system, Nervous system
儲存類別代碼
4.3 - Hazardous materials which set free flammable gases upon contact with water
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Yi Luan et al.
Organic letters, 13(9), 2510-2513 (2011-04-09)
Diazo esters, arylboranes, and carbamoyl imines undergo a 3-component Mannich condensation reaction. Catalyzed by Cu(II) salts, the reaction affords the corresponding isocyanate Mannich product: one that can be easily trapped in situ by other nucleophiles. The Mannich condensation corresponds to
A Ganesan
Mini reviews in medicinal chemistry, 6(1), 3-10 (2006-02-07)
Solid-phase synthesis is a powerful tool for achieving high-throughput chemistry. This review discusses recent diverse examples from my group: the solid-phase synthesis of unsymmetrical guanidines, polymer-supported versions of cyclooctadiene and 9-BBN, a triflate-like linker, the synthesis of tetrahydro-beta-carbolines by the
Jefferson D Revell et al.
Organic letters, 7(5), 831-833 (2005-02-25)
1,5-Cyclooctadiene was deprotonated under LICKOR conditions and reacted with Merrifield resin to afford an immobilized cyclooctadiene in high yield. This polymer is effective as a halogen scavenger, while hydroboration leads to a supported 9-BBN analogue. The latter exhibits similar regioselectivity
Rajesh Sardar et al.
Journal of the American Chemical Society, 133(21), 8179-8190 (2011-05-10)
We report a spectroscopic and microscopic investigation of the synthesis of gold nanoparticles (AuNPs) with average sizes of less than 5 nm. The slow reduction and AuNP formation processes that occur by using 9-borabicyclo[3.3.1]nonane (9-BBN) as a reducing agent enabled
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![9-硼双环[3.3.1]壬烷 溶液 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)
![9-硼双环[3.3.1]壬烷二聚体](/deepweb/assets/sigmaaldrich/product/structures/203/431/624973a6-aec1-4b23-b6c4-013285ac418c/640/624973a6-aec1-4b23-b6c4-013285ac418c.png)