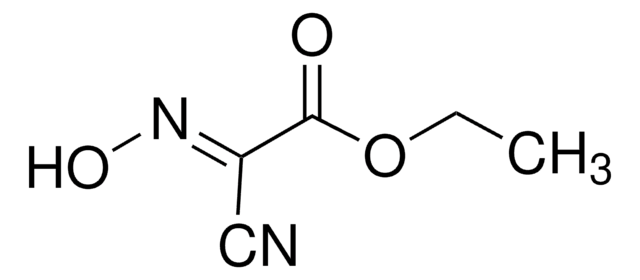
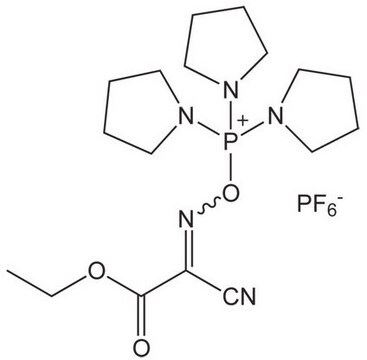
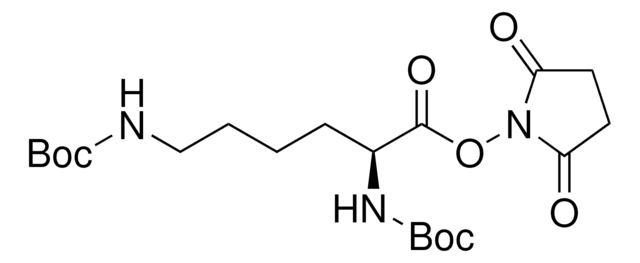
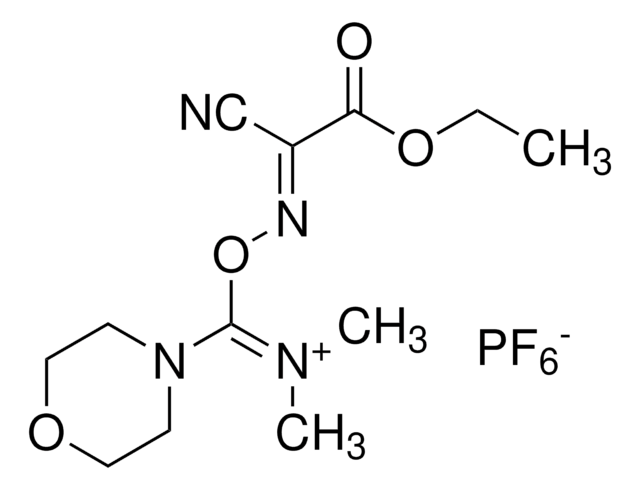
推荐产品
一般說明
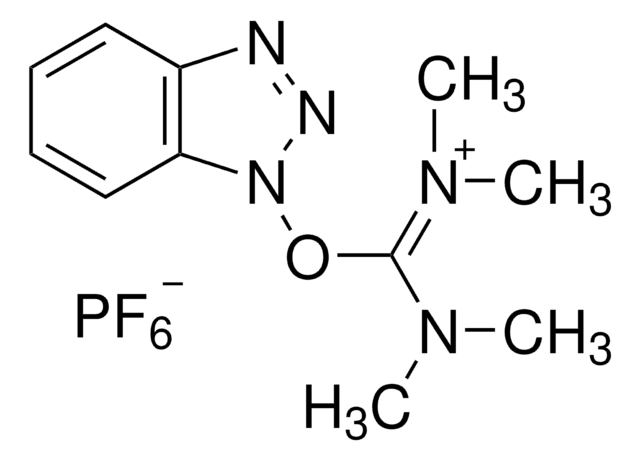
1-羟基-7-氮杂苯并三唑溶液,又称3H-[1,2,3]-三唑并[4,5-b]吡啶-3-醇,是一种苯并三唑类添加剂,常用作氨基酸和肽的偶联试剂。它还被用于最小化对映异构体生成。
應用
1-羟基-7-氮杂苯并三唑溶液被用作手性肽核酸的固相合成试剂。
其他說明
偶联添加剂,用于肽合成中的高效无外消旋偶联; 片段偶联。
訊號詞
Danger
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Eye Irrit. 2 - Flam. Liq. 3 - Repr. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
136.4 °F - closed cup
閃點(°C)
58 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Simone Di Micco et al.
Frontiers in chemistry, 8, 628609-628609 (2021-02-02)
The most severe outcome of COVID-19 infection is the development of interstitial pneumonia causing acute lung injury (ALI) and/or acute respiratory distress syndrome (ARDS), both responsible for the infected patients' mortality. ALI and ARDS are characterized by a leakage of
L.A. Carpino, A. El-Faham
Tetrahedron, 55, 6813-6813 (1999)
Hsuan-Ni Lin et al.
Journal of orthopaedic research : official publication of the Orthopaedic Research Society, 34(11), 1883-1893 (2016-02-27)
Fracture healing is regulated by a variety of inflammatory mediators and growth factors which act over time to regenerate the injured tissue. This study used a mouse femur fracture model to quantify the temporal expression pattern of lipid mediators, cytokines
Júlia García-Pindado et al.
Biopolymers, 109(10), e23112-e23112 (2018-03-13)
While revisiting biologically active natural peptides, the importance of the tryptophan residue became clear. In this article, the incorporation of this amino acid, brominated at different positions of the indole ring, into cyclic peptides was successfully achieved. These products demonstrated
Y. Nishiyama et al.
Tetrahedron Letters, 42, 8789-8789 (2001)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持