推荐产品
化驗
97%
形狀
solid
光學活性
[α]20/D +160°, c = 1 in ethanol
mp
56-58 °C (lit.)
官能基
ether
phenyl
儲存溫度
−20°C
SMILES 字串
CC(C)(C1=N[C@@H](CO1)c2ccccc2)C3=N[C@@H](CO3)c4ccccc4
InChI
1S/C21H22N2O2/c1-21(2,19-22-17(13-24-19)15-9-5-3-6-10-15)20-23-18(14-25-20)16-11-7-4-8-12-16/h3-12,17-18H,13-14H2,1-2H3/t17-,18-/m0/s1
InChI 密鑰
JTNVCJCSECAMLD-ROUUACIJSA-N
應用
不对称催化作用的 C2 对称配体。由于噁唑啉氮具有对各种金属的强亲合性,因此容易形成双齿配位络合物。
(+)-2,2′-Isopropylidenebis[(4R)-4-phenyl-2-oxazoline] in the presence of copper iodide, can catalyze the asymmetric cyclopropanation reaction of phenyliodonium ylides with alkenes to form cyclopropane α-amino acid esters.
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
其他客户在看
Benoît Moreau et al.
Journal of the American Chemical Society, 127(51), 18014-18015 (2005-12-22)
A highly enantioselective (up to 97.5% ee) and diastereoselective (95:5 dr trans/cis) Cu(I)-catalyzed cyclopropanation of alkenes using phenyliodonium ylide generated in situ from iodosobenzene and methyl nitroacetate is reported. The cyclopropanation took place with high enantioselectivity for a wide range
Pfaltz, A.
Accounts of Chemical Research, 26, 339-339 (1993)
Bolm, C.
Angewandte Chemie (International Edition in English), 30, 542-542 (1991)
Gant, T.G. Meyers, A.I.
Tetrahedron, 50, 2297-2297 (1994)
商品
C2-symmetric chiral bisoxazolines (BOX) ligands
C2-symmetric chiral bisoxazolines (BOX) ligands
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![(-)-2,2′-异亚丙基双[(4S)-4-苯基-2-噁唑啉] 97%](/deepweb/assets/sigmaaldrich/product/structures/297/720/a29f61c3-34e4-410c-acdd-241699b80af3/640/a29f61c3-34e4-410c-acdd-241699b80af3.png)
![2,2′-异亚丙基双[(4S)-4-叔丁基-2-噁唑啉] 99%](/deepweb/assets/sigmaaldrich/product/structures/334/357/19788a81-5365-46fd-978b-6b98382b1117/640/19788a81-5365-46fd-978b-6b98382b1117.png)
![2,2′-双[(4S)-4-苄基-2-噁唑啉] 98%](/deepweb/assets/sigmaaldrich/product/structures/139/783/42da3c77-52af-401b-8525-35d96415e284/640/42da3c77-52af-401b-8525-35d96415e284.png)
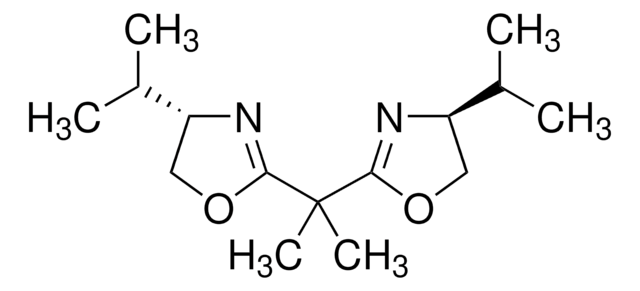
![2,6-双[(4S)-(-)-异丙基-2-噁唑啉-2-基]吡啶 99%](/deepweb/assets/sigmaaldrich/product/structures/452/550/7e22a7c6-e84a-4741-af9a-e40f05d8061c/640/7e22a7c6-e84a-4741-af9a-e40f05d8061c.png)
![[3aR-[2(3′aR*,8′aS*),3′aβ,8′aβ]]-(+)-2,2′-亚甲基双[3a,8a-二氢-8H-茚并[1,2-]噁唑] 98%](/deepweb/assets/sigmaaldrich/product/structures/134/031/294d2464-1571-4514-8e4c-c0cda1c1df7b/640/294d2464-1571-4514-8e4c-c0cda1c1df7b.png)
![2,6-双[(3aR,8aS)-(+)-8H-茚并[1,2-d]噁唑啉-2-基)吡啶 ≥94%](/deepweb/assets/sigmaaldrich/product/structures/123/619/565288e2-e1c9-4825-a440-17e786bc2c27/640/565288e2-e1c9-4825-a440-17e786bc2c27.png)
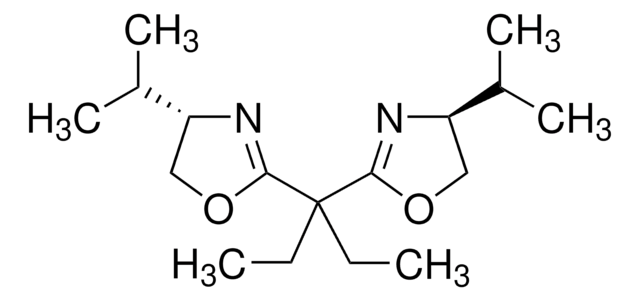
![2,2′-亚甲基双[(4S)-4-苯基-2-噁唑啉] 97%](/deepweb/assets/sigmaaldrich/product/structures/255/350/4403d4f8-c973-4da7-a5b6-2e93d1eacb10/640/4403d4f8-c973-4da7-a5b6-2e93d1eacb10.png)
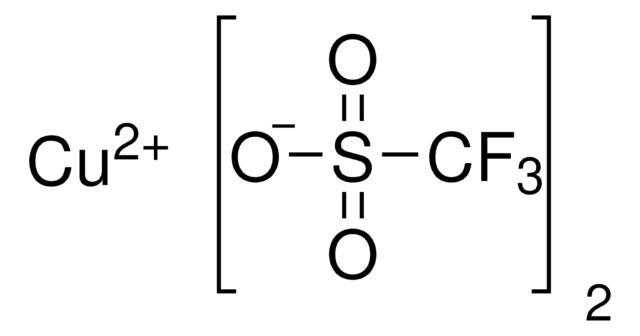

![(4S)-(+)-苯基-α-[(4S)-苯基噁唑烷-2-亚基]-2-噁唑啉-2-乙腈 97%](/deepweb/assets/sigmaaldrich/product/structures/117/759/d4e6e882-8577-4dcf-84fe-506633ae811a/640/d4e6e882-8577-4dcf-84fe-506633ae811a.png)
![2,6-双[(4R)-(+)-异丙基-2-噁唑啉-2-基]吡啶 99%](/deepweb/assets/sigmaaldrich/product/structures/349/609/8673c46e-368a-47a6-a9bd-52bbe13d490a/640/8673c46e-368a-47a6-a9bd-52bbe13d490a.png)