所有图片(1)
About This Item
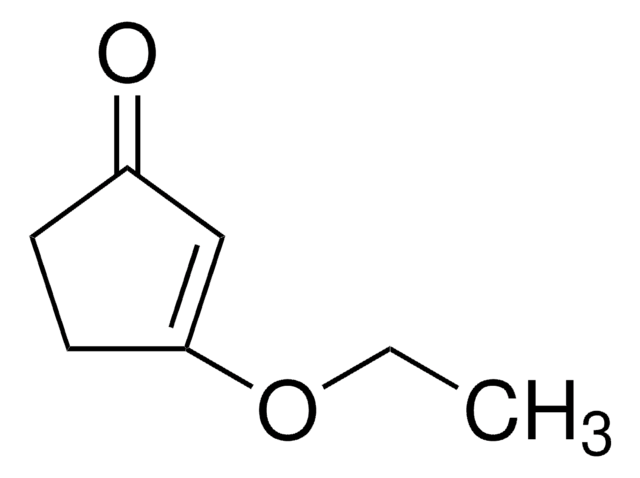
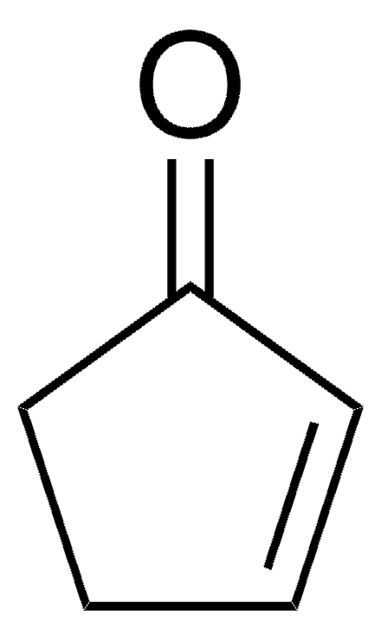
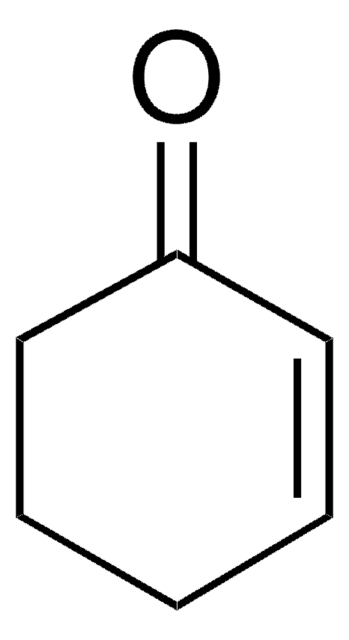
线性分子式:
CH3OC5H5(=O)
CAS号:
分子量:
112.13
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
99%
形狀
solid
mp
49-53 °C (lit.)
官能基
ether
ketone
SMILES 字串
COC1=CC(=O)CC1
InChI
1S/C6H8O2/c1-8-6-3-2-5(7)4-6/h4H,2-3H2,1H3
InChI 密鑰
DTWCFCILAJVNPE-UHFFFAOYSA-N
一般說明
3-Methoxy-2-cyclopenten-1-one (3-methoxycyclopent-2-enone) is a 3-methoxycycloalk-2- enone.
應用
用于合成环戊烷抗生素 (+)-海黍子酮的原料。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Patrick Y Toullec et al.
Proceedings of the National Academy of Sciences of the United States of America, 101(16), 5810-5814 (2004-04-09)
The enantioselective formation of a quaternary stereogenic center coinciding with a hydroxylation process is a very rare reaction from a homogeneous catalysis point of view. Indeed, to our knowledge, no asymmetric transition-metal-catalyzed direct hydroxylation has been reported before. We describe
Tetrahedron, 47, 173-173 (1991)
Discovery of orally efficacious melanin-concentrating hormone receptor-1 antagonists as antiobesity agents. Synthesis, SAR, and biological evaluation of bicyclo [3.1. 0] hexyl ureas.
McBriar MD, et al.
Journal of Medicinal Chemistry, 49(7), 1202-1207 (2012)
Yeonjoon Kim et al.
Bioorganic & medicinal chemistry letters, 24(13), 2807-2810 (2014-05-24)
3-Alkyl-2-aryl-2-cyclopenten-1-one oxime derivatives (1) were studied as a novel class of inhibitors of tumor necrosis factor α (TNF-α) with regard to synthesis and in vitro SAR inhibition of TNF-α. The in vitro IC50 values of these compounds in rat and
Gamal A I Moustafa et al.
The Journal of organic chemistry, 77(2), 1202-1207 (2012-01-03)
The alkylation of dienolates generated from 3-methoxycycloalk-2-enones having a 3'-hydroxyl alkenyl chain provides the corresponding quaternized cycloalkenones in a highly diastereoselective manner. The high degree of stereocontrol in the α-quaternization possibly implies intervention of a rigid chelating transition state that
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门