推荐产品
品質等級
化驗
99%
形狀
solid
mp
158-160 °C (lit.)
溶解度
chloroform: soluble 25 mg/mL, clear, colorless to yellow
官能基
sulfone
SMILES 字串
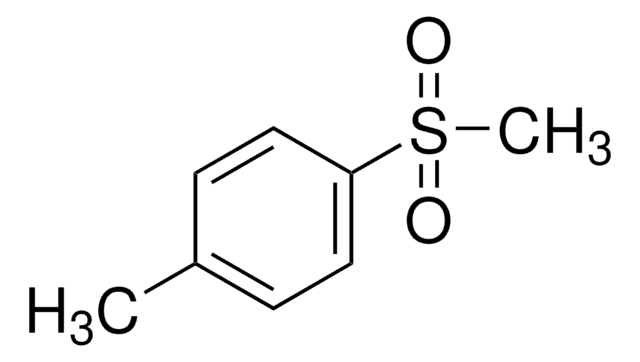
Cc1ccc(cc1)S(=O)(=O)c2ccc(C)cc2
InChI
1S/C14H14O2S/c1-11-3-7-13(8-4-11)17(15,16)14-9-5-12(2)6-10-14/h3-10H,1-2H3
InChI 密鑰
WEAYCYAIVOIUMG-UHFFFAOYSA-N
一般說明
Di-p-tolyl sulfone is a di-p-substituted diaryl sulfone. Its synthesis by the sulfonylation of toluene with p-toluenesulfonic acid (TsOH) in the presence of polystyrene supported aluminium triflate (Ps-Al(OTf)3) catalyst has been reported along with its NMR and IR spectra. The gas-phase heats of formation of di-p-tolyl sulfone has been studied. The kinetics and thermodynamics of sulphuric acid assisted cleavage of di-p-tolyl sulfone has been invesitigated.
應用
Di-p-tolyl sulfone may be used in the synthesis of isomeric p-tolylpyridines (α , β and γ ) by photochemical decomposition.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Para tolylation of pyridine by photolysis of di-p-tolyl sulfone and related compounds.
Nakabayashi T, et al.
Bulletin of the Chemical Society of Japan, 50(9), 2491-2492 (1977)
Studies in the thermochemistry of sulphones. Part 6.-Heats of combustion, fusion, vaporization and atomization of six aromatic and two allylic sulphones.
Mackle H and O'Hare PAG.
Transactions of the Faraday Society, 57, 1521-1526 (1961)
Polystyrene Supported Al (OTf)3: a Stable, Efficient, Selective, and Reusable Catalyst for Sulfonylation of Arenes with Sulfonic Acids.
Boroujeni, KP.
Bull. Korean Chem. Soc., 31(7), 1887-1890 (2010)
Kinetics and thermodynamics of sulfuric acid-mediated cleavage of substituted diaryl sulfones.
Ward RS, et al.
Journal of Surfactants and Detergents, 4(2), 185-190 (2001)
Kazumasa Okamoto et al.
Scientific reports, 10(1), 19823-19823 (2020-11-15)
Dimer radical ions of aromatic molecules in which excess charge is localized in a pair of rings have been extensively investigated. While dimer radical cations of aromatics have been previously produced in the condensed phase, the number of molecules that
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持