About This Item
推荐产品
化驗
97%
形狀
powder
mp
143-145 °C (lit.)
SMILES 字串
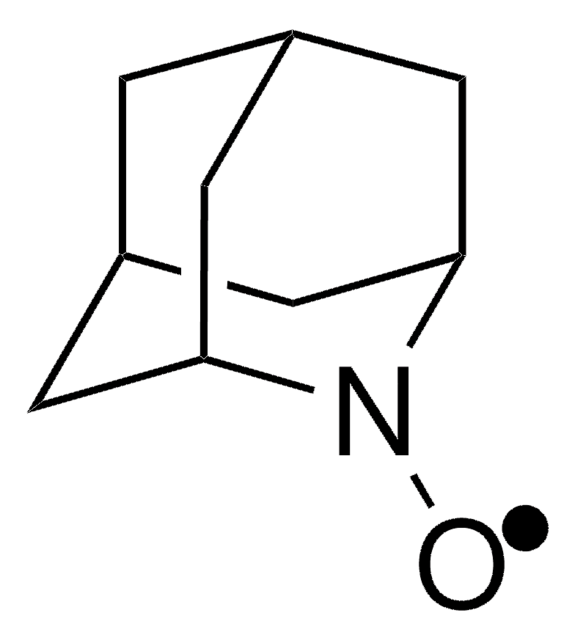
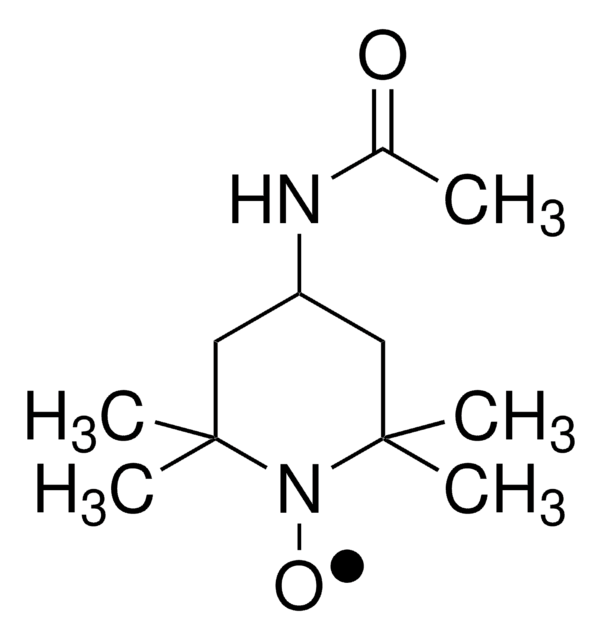
CC(=O)NC1CC(C)(C)N([O])C(C)(C)C1
InChI
1S/C11H21N2O2/c1-8(14)12-9-6-10(2,3)13(15)11(4,5)7-9/h9H,6-7H2,1-5H3,(H,12,14)
InChI 密鑰
UXBLSWOMIHTQPH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
商品
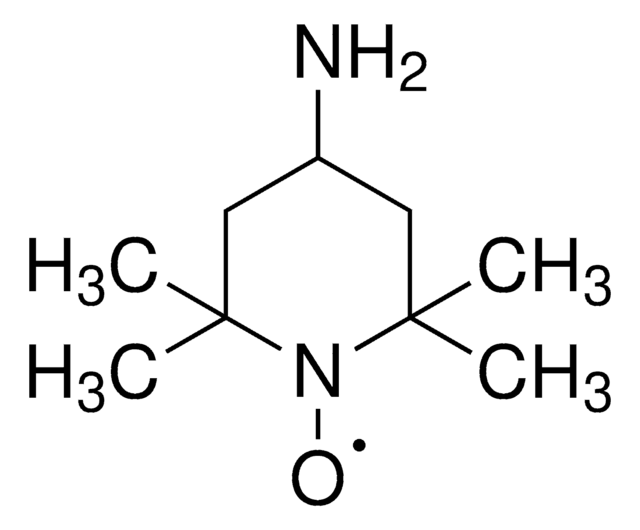
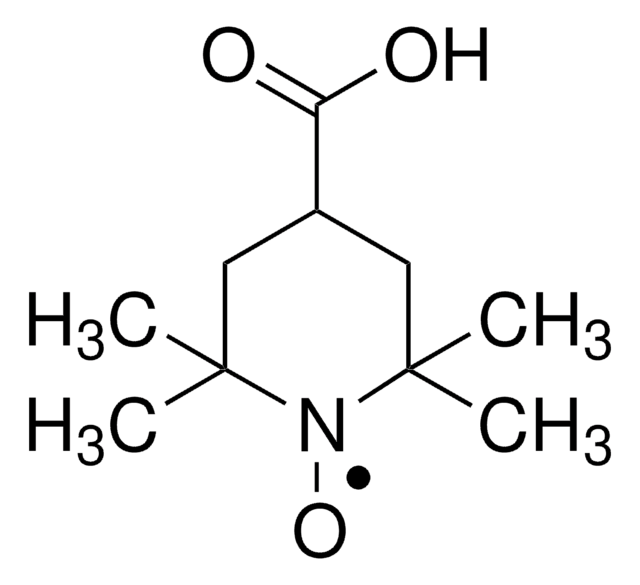
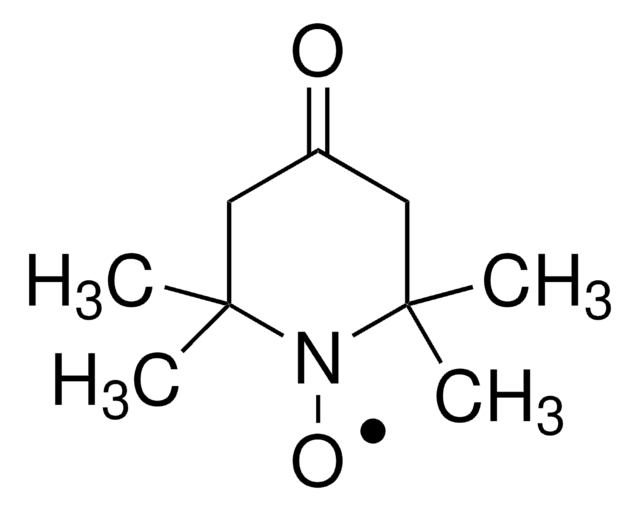
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门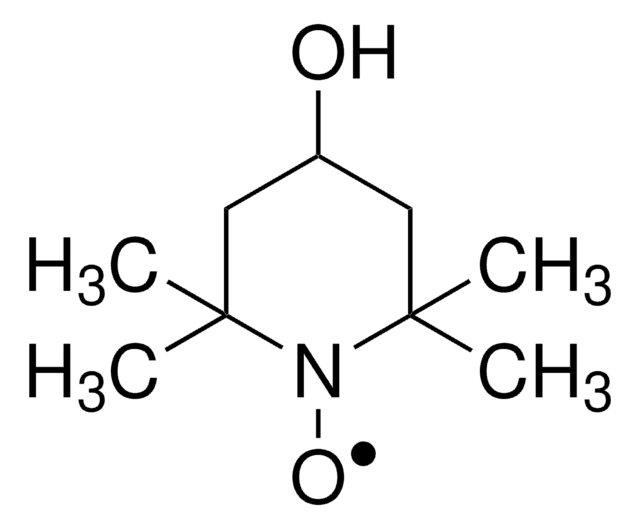

![9-氮杂双环[3.3.1]壬烷N-氧基 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)