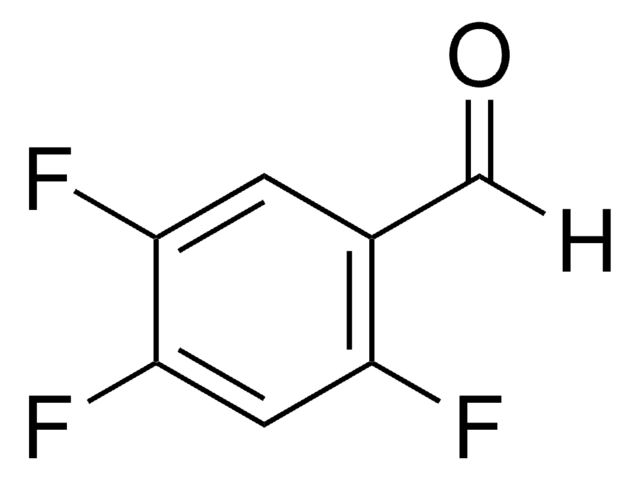
推荐产品
化驗
97%
形狀
liquid
折射率
n20/D 1.469 (lit.)
bp
178 °C (lit.)
密度
1.525 g/mL at 25 °C (lit.)
官能基
aldehyde
fluoro
SMILES 字串
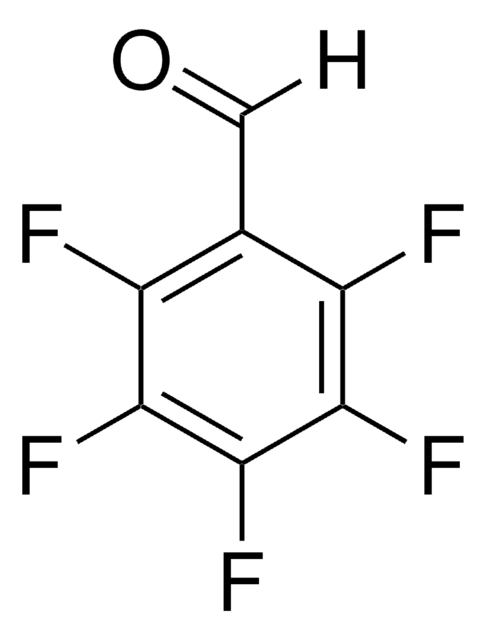
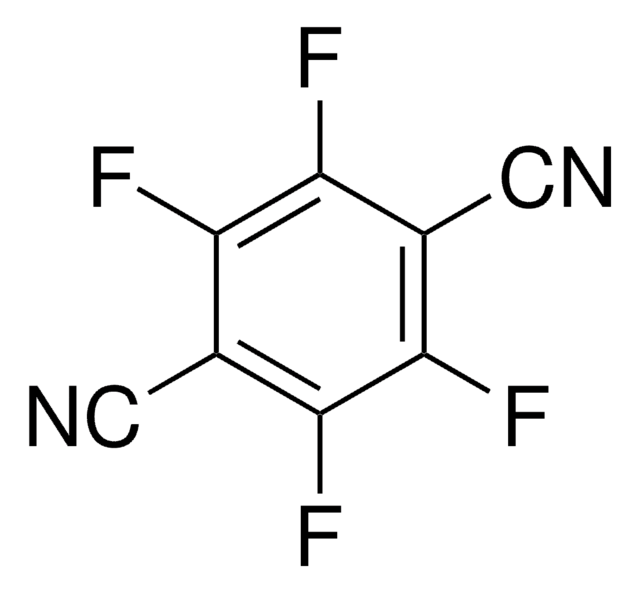
Fc1cc(F)c(F)c(C=O)c1F
InChI
1S/C7H2F4O/c8-4-1-5(9)7(11)3(2-12)6(4)10/h1-2H
InChI 密鑰
YIRYOMXPMOLQSO-UHFFFAOYSA-N
一般說明
2,3,5,6-Tetrafluorobenzaldehyde is a polysubstituted benzaldehyde and was evaluated as a substrate of PmHNL (Prunus mume hydroxynitrile lyase). Reaction of 2,3,5,6-tetrafluorobenzaldehyde with dipyrromethane was reported.
應用
2,3,5,6-Tetrafluorobenzaldehyde was used in the preparation of 1,3-bis(2,4,6-trimethylphenyl)-2-(2,3,5,6-tetrafluorophenyl)imidazolidine and 1,3-dimethyl-2-(2,3,5,6-tetrafluorophenyl)imidazolidine.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
165.2 °F - closed cup
閃點(°C)
74 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
A new (R)-hydroxynitrile lyase from< i> Prunus mume</i>: asymmetric synthesis of cyanohydrins.
Nanda S, et al.
Tetrahedron, 61(46), 10908-10916 (2005)
Effects of aldehyde or dipyrromethane substituents on the reaction course leading to meso-substituted porphyrins.
Geier III, et al.
Tetrahedron, 60(50), 11435-11444 (2004)
Gregory W Nyce et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 10(16), 4073-4079 (2004-08-19)
The synthesis of N-heterocyclic carbene (NHC) adducts by condensation of diamines with appropriately substituted benzaldehydes is described. This simplified approach provides the NHC adduct without first having to generate the carbene followed by its protection. These adducts undergo thermal deprotection
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门