推荐产品
化驗
97%
折射率
n20/D 1.490 (lit.)
bp
35-37 °C/1.3 mmHg (lit.)
密度
1.489 g/mL at 25 °C (lit.)
儲存溫度
−20°C
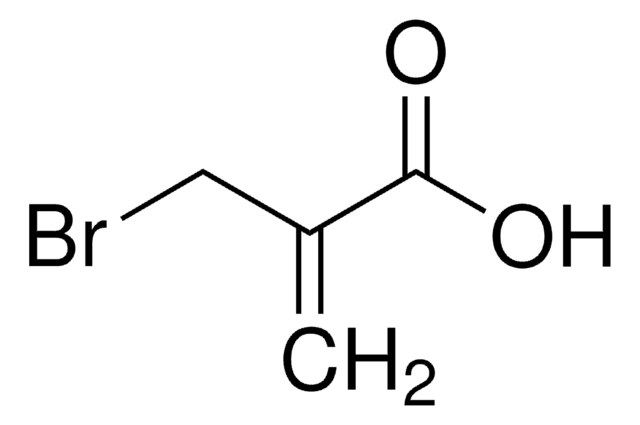
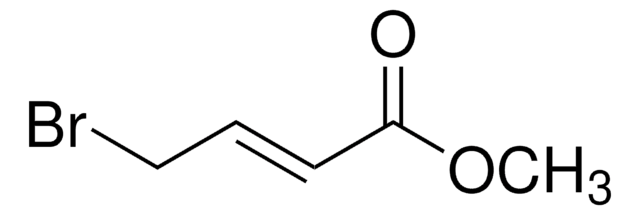
SMILES 字串
COC(=O)C(=C)CBr
InChI
1S/C5H7BrO2/c1-4(3-6)5(7)8-2/h1,3H2,2H3
InChI 密鑰
CFTUQSLVERGMHL-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Methyl (2-bromomethyl)acrylate (MBrMA) may be used as chain transfer agents in the emulsion polymerization of methyl methacrylate (MMA) and styrene. MBrMA can undergo nucleophilic substitution of carboxylic acid to form methyl 2-(acyloxymethyl)acrylates.
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
172.4 °F - closed cup
閃點(°C)
78 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Bunichiro Yamada, Shuzo Aoki, Radical polymerization and copolymerization of methyl 2-(acyloxymethyl)acrylate as hindered 2-substituted acrylate
Kobatake S, et al.
Polymer, 36(2), 413-419 (1995)
Mukund P Sibi et al.
Tetrahedron, asymmetry, 17(4), 516-519 (2006-06-27)
We have investigated the effect of nitrogen protecting groups in radical addition trapping experiments leading to beta(2)-amino acids. Of the three N-protecting groups examined, the phthalimido group was optimal with respect to both yields and enantioselectivity. Additionally, radical additions to
Use of methyl 2-(bromomethyl) acrylate as a chain-transfer agent to yield functionalized macromonomers via conventional and living radical polymerizations.
Bon, SAF, et al.
Macromolecules, 33(16), 5819-5824 (2000)
Thomas Mendgen et al.
Bioorganic & medicinal chemistry letters, 20(19), 5757-5762 (2010-08-24)
The enzyme MurA performs an essential step in peptidoglycan biosynthesis and is therefore a target for the discovery of novel antibacterial compounds. We report here the inhibition of MurA by natural products from tulips (tulipalines and tuliposides), and the structure-activity
Fabrice Denes et al.
The Journal of organic chemistry, 72(2), 398-406 (2007-01-16)
Enantioenriched 3,4-disubstituted beta-prolines have been prepared with a high diastereocontrol through a carbometalation reaction or through a domino Michael addition/carbometalation reaction.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门