推荐产品
化驗
98%
形狀
liquid
光學活性
[α]21/D +96.1°, c = 2 in chloroform
折射率
n20/D 1.461 (lit.)
bp
93-95 °C/0.1 mmHg (lit.)
密度
1.15 g/mL at 25 °C (lit.)
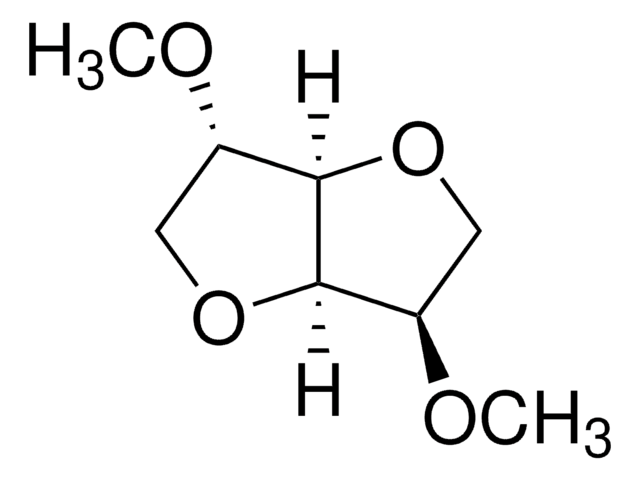
SMILES 字串
CO[C@H]1COC2[C@@H](COC12)OC
InChI
1S/C8H14O4/c1-9-5-3-11-8-6(10-2)4-12-7(5)8/h5-8H,3-4H2,1-2H3/t5-,6+,7-,8-/m1/s1
InChI 密鑰
MEJYDZQQVZJMPP-ULAWRXDQSA-N
正在寻找类似产品? 访问 产品对比指南
應用
异山梨醇二甲醚(DMI)是一种可持续溶剂,广泛用于各种化妆品和药物配方中。
通用应用:
通用应用:
- DMI 可用作环辛烯环氧化的替代绿色溶剂,异麦芽酮糖衍生醛与 α,β-不饱和酮的贝里斯-希尔曼反应,也可用于固相合成。
- 它可以用作水性涂料的聚结剂,在失水阶段充当共溶剂,在成膜过程中充当聚结剂。
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
240.8 °F - closed cup
閃點(°C)
116 °C - closed cup
個人防護裝備
Eyeshields, Gloves
其他客户在看
H Zia et al.
Pharmaceutical research, 8(4), 502-504 (1991-04-01)
Dimethyl isosorbide (DMI), which is currently under investigation for its potential use as a pharmaceutical vehicle and drug permeation enhancer, is a water-miscible liquid with relatively low viscosity. The solubilization behavior of DMI as a cosolvent for nonpolar drugs was
A Otto et al.
Skin pharmacology and physiology, 21(6), 326-334 (2008-10-04)
In this study the effect of 2 penetration modifiers, dimethyl isosorbide (DMI) and diethylene glycol monoethyl ether (DGME) on the skin delivery of hydroquinone (HQ), salicylic acid (SA) and octadecenedioic acid (DIOIC) was investigated. Ten percent DMI and DGME were
Eco-friendly solvents and amphiphilic catalytic polyoxometalate nanoparticles: a winning combination for olefin epoxidation.
Mouret A, et al.
Green Chemistry, 16(1), 269-278 (2014)
Bola D Majekodunmi et al.
Pharmaceutical development and technology, 12(6), 609-620 (2007-12-29)
The chemical stability of benzoyl peroxide (BPO) was studied in solutions and gels. The solutions (1% w/v) were prepared in single solvents (alcohol USP, isopropyl alcohol USP, ethyl benzoate, C12-15 alkyl benzoate, dimethyl isosorbide, propylene carbonate, and acetone) and in
HMF derivatives as platform molecules: aqueous Baylis-Hillman reaction of glucosyloxymethyl-furfural towards new biobased acrylates.
Tan JN, et al.
Royal Society of Chemistry Advances, 3(39), 17649-17653 (2013)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门