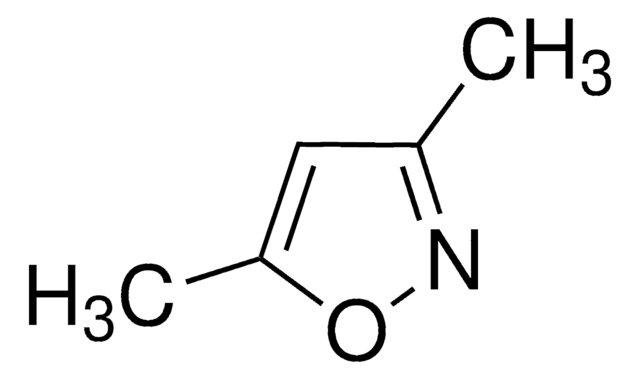
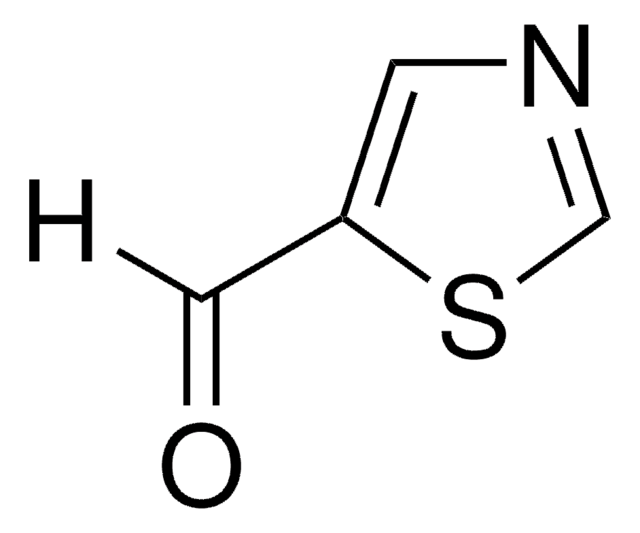
推荐产品
化驗
98%
折射率
n20/D 1.425 (lit.)
bp
69-70 °C (lit.)
mp
−87-−84 °C (lit.)
密度
1.05 g/mL at 25 °C (lit.)
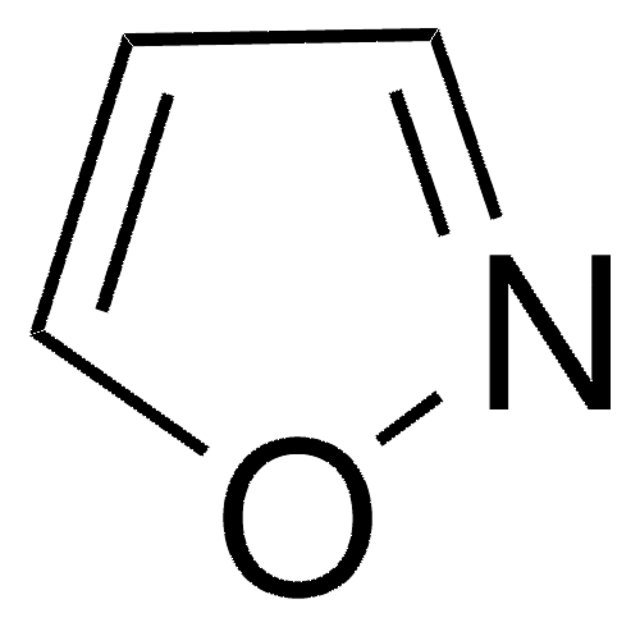
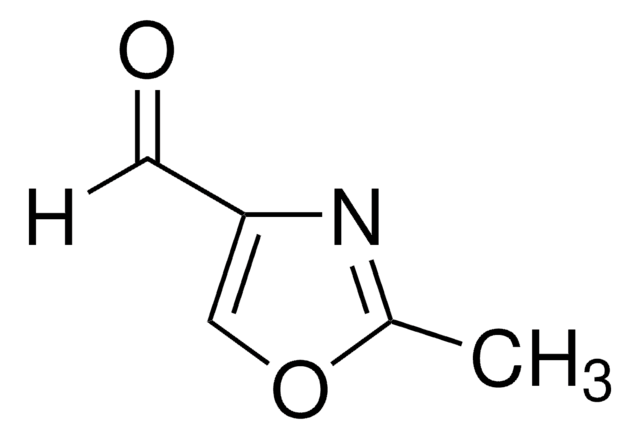
SMILES 字串
c1cocn1
InChI
1S/C3H3NO/c1-2-5-3-4-1/h1-3H
InChI 密鑰
ZCQWOFVYLHDMMC-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
恶唑是一大类杂环芳香化合物的母体分子。它是弱碱性的,可作为缺电子二烯用于Diels-Alder环加成反应。它可进行硝化、磺化、卤化、Friedel-Crafts烷化和酰化反应。
應用
Oxazole can be used:
- in the intramolecular Diels–Alder (IMDA) cycloaddition reaction to synthesis natural products
- as a precursor in the ring opening, nucleophilic addition and recyclization as well as [2 + 2], [3 + 2], and [4 + 2] cycloaddition reactions
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Flam. Liq. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
66.2 °F - closed cup
閃點(°C)
19 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Maryna V Kachaeva et al.
Computational biology and chemistry, 74, 294-303 (2018-04-27)
Based on modern literature data about biological activity of E7010 derivatives, a series of new sulfonamides as potential anticancer drugs were rationally designed by QSAR modeling methods Сlassification learning QSAR models to predict the tubulin polymerization inhibition activity of novel
Haseen Ahmad et al.
European journal of medicinal chemistry, 208, 112759-112759 (2020-09-05)
Oxazole derivatives are important medicinal compounds which are inhibitors of various enzymes such as NPP1, NPP2, NPP3, tyrosine kinase, dipeptidyl-peptidase IV, cyclooxygenase-2, and protein tyrosine phosphatase. In this study, an extensive range of new biologically active biphenyl oxazole derivatives was
Dawid Siodłak et al.
The journal of physical chemistry. B, 118(9), 2340-2350 (2014-02-18)
Oxazole ring occurs in numerous natural peptides, but conformational properties of the amino acid residue containing the oxazole ring in place of the C-terminal amide bond are poorly recognized. A series of model compounds constituted by the oxazole-amino acids occurring
Ilya Lyagin et al.
Molecules (Basel, Switzerland), 24(13) (2019-06-30)
Mycotoxins are highly dangerous natural compounds produced by various fungi. Enzymatic transformation seems to be the most promising method for detoxification of mycotoxins. This review summarizes current information on enzymes of different classes to convert various mycotoxins. An in-depth analysis
Kristjan Bloudoff et al.
Proceedings of the National Academy of Sciences of the United States of America, 114(1), 95-100 (2016-12-21)
Nonribosomal peptide synthetases (NRPSs) are a family of multidomain, multimodule enzymes that synthesize structurally and functionally diverse peptides, many of which are of great therapeutic or commercial value. The central chemical step of peptide synthesis is amide bond formation, which
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门