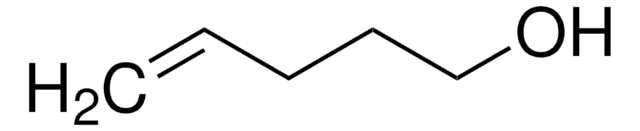
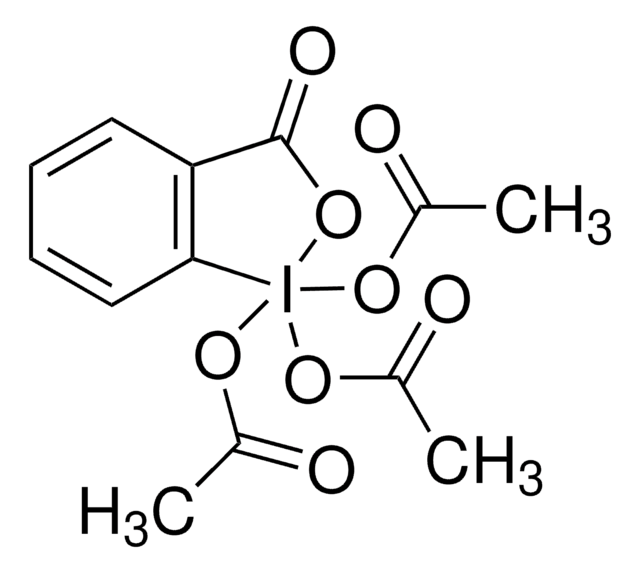
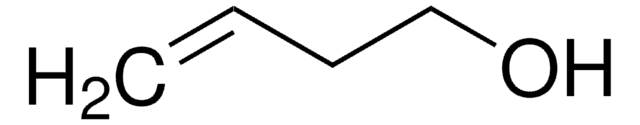
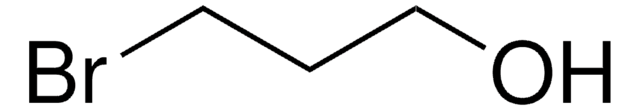
推荐产品
應用
烯丙基三甲基硅烷是一种将烯丙基引入氯化酰基、醛类、酮类、亚胺离子、烯酮类以及与其他碳亲电试剂交叉偶联的通用试剂。它被用作Hosomi−Sakurai反应中的试剂。
訊號詞
Danger
危險聲明
危險分類
Flam. Liq. 2 - Skin Irrit. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
60.8 °F - closed cup
閃點(°C)
16 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Allyltrimethylsilane
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2001)
Armando Ramirez et al.
Organic letters, 7(21), 4617-4620 (2005-10-08)
[reaction: see text] The annulation reactions of alkenes with peroxycarbenium ions enable the synthesis of a variety of functionalizable 1,2-dioxolanes. Triethysilyl-protected peroxycarbenium ions proved to be optimal for the annulation reaction. Using this method, plakinic acid analogues can be synthesized
Allylation of Imines with Allyltrimethylsilane and Experimental Evidences for a Fluoride-Triggered Autocatalysis Mechanism of the Sakurai- Hosomi Reaction
Wang D, et al.
The Journal of Organic Chemistry, 64(12), 4233-4237 (1999)
Shoji Kobayashi et al.
Carbohydrate research, 343(3), 443-452 (2007-12-11)
An efficient route to the trans-fused tetrahydrooxepin corresponding to the E ring of ciguatoxin was developed. Wide screening of allylation reactions of sulfur or fluoro-substituted tetrahydrooxepin revealed that the optimum method for obtaining the beta-allylation product selectively was the use
Allyltrimethylsilane
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
商品
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门