About This Item
推荐产品
化驗
97%
形狀
powder
反應適用性
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Carbonylations
reagent type: ligand
reaction type: Ene Reaction
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Stille Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Tsuji-Trost Reaction
環保替代產品特色
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
181-182 °C (dec.) (lit.)
官能基
phosphine
環保替代類別
SMILES 字串
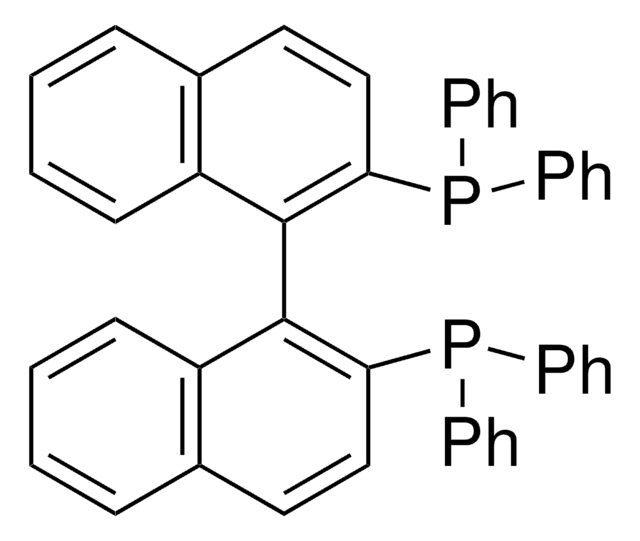
[Fe].[CH]1[CH][CH][C]([CH]1)P(c2ccccc2)c3ccccc3.[CH]4[CH][CH][C]([CH]4)P(c5ccccc5)c6ccccc6
InChI
1S/2C17H14P.Fe/c2*1-3-9-15(10-4-1)18(17-13-7-8-14-17)16-11-5-2-6-12-16;/h2*1-14H;
InChI 密鑰
HPXNTHKXCYMIJL-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)