推荐产品
化驗
95%
折射率
n20/D 1.406 (lit.)
bp
85 °C (lit.)
密度
0.87 g/mL at 25 °C (lit.)
官能基
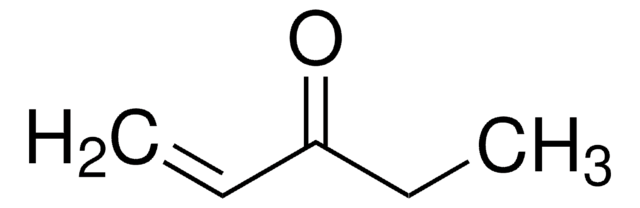
ketone
SMILES 字串
CC(=O)C#C
InChI
1S/C4H4O/c1-3-4(2)5/h1H,2H3
InChI 密鑰
XRGPFNGLRSIPSA-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
3-丁炔-2-酮在手性氨基膦的催化下可与邻甲苯磺酰基苯丙二酸酯发生不对称的双迈克尔反应生成二氢吲哚。它可与含氮的束缚二酸进行双迈克尔反应生成胡椒酸衍生物。
應用
3-丁炔-2-酮可用于克罗烷二萜(+/-)-沙卡菌素的合成。它可作为氯化镓(III)介导的立体选择性共轭芳基化的底物用于生成(E)-α,β-不饱和酮。
訊號詞
Danger
危險分類
Acute Tox. 2 Oral - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
30.2 °F - closed cup
閃點(°C)
-1 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Synlett, 809-809 (2007)
R B Grossman et al.
Organic letters, 3(25), 4027-4030 (2001-12-12)
[reaction: see text] The putative structure of the naturally occurring clerodane diterpenoid (+/-)-sacacarin has been prepared in only 10 steps, six of which are C-C bond-forming steps, in a chemo-, regio-, and diastereoselective manner. The key part of the synthesis
F Hughes et al.
Organic letters, 3(18), 2911-2914 (2001-09-01)
[reaction: see text]. Nitrogen-containing tethered diacids, easily prepared by reductive alkylation of diethyl aminomalonate or ethyl cyanoglycinate, undergo double Michael reactions with 3-butyn-2-one to give highly functionalized and substituted piperidines (pipecolic acid derivatives) with surprisingly high stereoselectivity. The heterocyclic double
San N Khong et al.
Molecules (Basel, Switzerland), 17(5), 5626-5650 (2012-05-15)
The bisphosphine-catalyzed double-Michael addition of dinucleophiles to electron-deficient acetylenes is an efficient process for the synthesis of many nitrogen-containing heterocycles. Because the resulting heterocycles contain at least one stereogenic center, this double-Michael reaction would be even more useful if an
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门