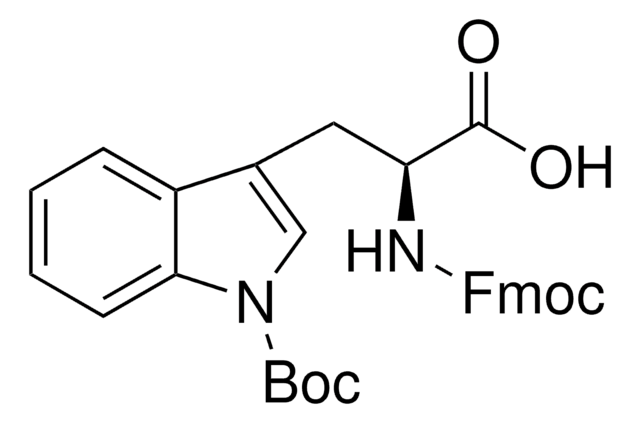
所有图片(1)
About This Item
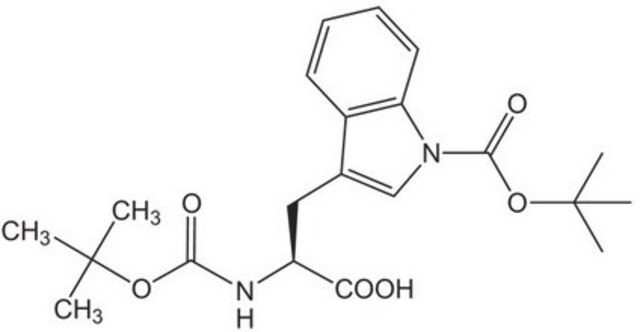
经验公式(希尔记法):
C16H20N2O4
CAS号:
分子量:
304.34
Beilstein:
39677
EC號碼:
MDL號碼:
分類程式碼代碼:
12352209
eCl@ss:
32160406
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
≥99.0% (TLC)
光學活性
[α]20/D −20±1°, c = 1% in DMF
反應適用性
reaction type: Boc solid-phase peptide synthesis
mp
136 °C (dec.) (lit.)
應用
peptide synthesis
SMILES 字串
CC(C)(C)OC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O
InChI
1S/C16H20N2O4/c1-16(2,3)22-15(21)18-13(14(19)20)8-10-9-17-12-7-5-4-6-11(10)12/h4-7,9,13,17H,8H2,1-3H3,(H,18,21)(H,19,20)/t13-/m0/s1
InChI 密鑰
NFVNYBJCJGKVQK-ZDUSSCGKSA-N
正在寻找类似产品? 访问 产品对比指南
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Bailey T , et al. et al.
Science of Synthesis: Houben-Weyl Methods of Molecular Transformations, 21 (2014)
Peroxidase catalyzed nitration of tryptophan derivatives
Sala A, et al.
European Journal of Biochemistry, 271(13), 2841-2852 (2004)
R Vespalec et al.
Electrophoresis, 19(2), 276-281 (1998-04-21)
An equation for the calculation of electrophoretic mobility of kinetically labile complexes originating in solutions during the chiral discrimination process is derived. The mobility of the complex is calculated from that of a fully ionized racemic compound, measured in absence
Y Kato et al.
Biochemical and biophysical research communications, 234(1), 82-84 (1997-05-08)
The aim of this study was to clarify the mechanism of loss of Trp residues in proteins exposed to peroxynitrite. The Trp residues in bovine serum albumin and collagen IV were decreased by peroxynitrite treatment. To identify the degradation products
R Magous et al.
Biochimica et biophysica acta, 845(2), 158-162 (1985-05-30)
Benzotript (N-p-chlorobenzoyl-L-tryptophan) has been shown to be a receptor-antagonist in vivo and in vitro for peptides from the gastrin family. In the present study, we examine tryptophan, and some of its N- and C-acylated derivatives, as well as some phenylalanine
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门