推荐产品
品質等級
化驗
96%
形狀
solid
mp
152-154 °C (lit.)
官能基
chloro
SMILES 字串
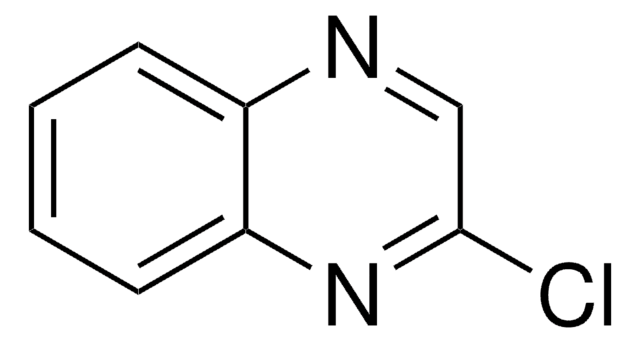
Clc1nc2ccccc2nc1Cl
InChI
1S/C8H4Cl2N2/c9-7-8(10)12-6-4-2-1-3-5(6)11-7/h1-4H
InChI 密鑰
SPSSDDOTEZKOOV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2,3-Dichloroquinoxaline reacts with 6-aminothiouracil in ethanol/TEA to form 6-amino-2-(3-chloroquinoxalin-2-ylthio)pyrimidin-4(3H)-one. It reacts with cholest-5-en-3-one semicarbazone/thiosemicarbazone to form steriodal cholest-5-en-3-oxazolo and thiazoloquinoxaline.
應用
2,3-Dichloroquinoxaline was used in the synthesis of mono and 2,3-disubstituted quinoxalines. It was used in solid phase synthesis of HPLC chiral stationary phase containing the N,N′-dialkyl-2,3-diaminoquinoxaline group as a linking structure.
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Performance of a new HPLC chiral stationary phase derived from N-(3, 5-dinitrobenzoyl)-D-alpha-phenylglycine with a quinoxaline branching unit.
Forjan DM, et al.
Acta Chromatographica , 17, 97-97 (2006)
K Shiva Kumar et al.
Bioorganic & medicinal chemistry, 20(5), 1711-1722 (2012-02-10)
A direct and single-step method has been developed for the synthesis of mono and 2,3-disubstituted quinoxalines by using a AlCl(3) induced (hetero)arylation of 2,3-dichloroquinoxaline. Both symmetrical and unsymmetrical 2,3-disubstituted quinoxalines can be prepared conveniently by using this method under appropriate
Shadia A Galal et al.
European journal of medicinal chemistry, 46(1), 327-340 (2010-12-15)
The reaction of o-phenylene diamine and ethyl oxamate is reinvestigated and led to 3-aminoquinoxalin-2(1H)-one rather than benzimidazole-2-carboxamide as was previously reported. The structure of the obtained quinoxaline has been confirmed by X-ray. The anti-tumor activity of synthesized quinoxalines 1-21 has
A A Abu-Hashem et al.
European journal of medicinal chemistry, 45(5), 1976-1981 (2010-02-13)
Treatment of 6-aminothiouracil (1) with 2,3-dichloroquinoxaline (2) in ethanol/TEA afforded 6-amino-2-(3-chloroquinoxalin-2-ylthio)pyrimidin-4(3H)-one (3), which was refluxed in DMF to give 2-aminopyrimido[2',1':2,3]thiazolo[4,5-b]quinoxaline-4-one (4). Compound 4 was utilized as a key intermediate for the synthesis of a new pyrimido[2',1':2,3]thiazolo[4,5-b]quinoxaline derivatives 5-14via the reaction
Salman Ahmad Khan et al.
European journal of medicinal chemistry, 43(10), 2257-2261 (2008-04-29)
Some heterocyclic systems namely cholest-5-en-7-thiazolo[4,5-b]quinoxaline-2-yl-hydrazone] were synthesized by the reaction of cholest-5-en-7-one-thiosemicarbazone with 2,3-dichloroquinoxaline at 80 degrees C in high yield. The thiosemicarbazone derivatives were obtained by the condensation of the thiosemicarbazide with steroidal ketones. All the compounds have been
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门