D1515
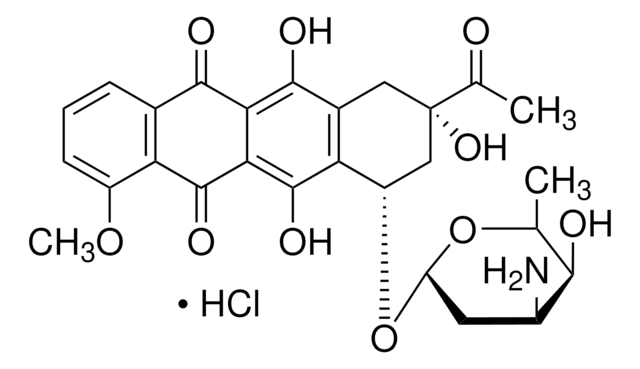
Doxorubicin hydrochloride
98.0-102.0% (HPLC)
Synonym(s):
Adriamycin, DOX, Hydroxydaunorubicin hydrochloride
About This Item
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
98.0-102.0% (HPLC)
form
powder
mp
216 °C (dec.) (lit.)
solubility
water: 50.0-52.0 mg/mL, clear, orange to red
antibiotic activity spectrum
viruses
Mode of action
DNA synthesis | interferes
storage temp.
2-8°C
SMILES string
Cl[H].COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO
InChI
1S/C27H29NO11.ClH/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34;/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3;1H/t10-,13-,15-,17-,22+,27-;/m0./s1
InChI key
MWWSFMDVAYGXBV-RUELKSSGSA-N
Gene Information
human ... TOP2A(7153)
Looking for similar products? Visit Product Comparison Guide
General description
Doxorubicin also leads to the generation of reactive oxygen species (ROS), which further damage DNA, proteins, and membranes. It exerts cytotoxic, antitumor, anticancer, and antineoplastic activity and finds application in cancer, metabolomics, cell biology, and biochemical research.
Application
- in cell viability assays
- MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay
- as a drug in polymeric PLGA-based microparticulate drug delivery
- to develop doxorubicin-resistant HepG2 cells (HepG2-DR) and K562 cells (K562-DR)
Biochem/physiol Actions
Features and Benefits
- High-quality antibiotic suitable for multiple research applications
- Ideal for Cell Biology, Metabolomics, and Biochemical research.
Preparation Note
Storage and Stability
Other Notes
comparable product
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 1B - Muta. 1B - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Quinolones are a key group of antibiotics that interfere with DNA synthesis by inhibiting topoisomerase, most frequently topoisomerase II (DNA gyrase), an enzyme involved in DNA replication.
Graphene oxide is a unique material that can be viewed as a single monomolecular layer of graphite with various oxygen containing functionalities such as epoxide, carbonyl, carboxyl and hydroxyl groups.
We presents an article on ABC Transporters and Cancer Drug Resistance
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service