P8833
Puromycin dihydrochloride from Streptomyces alboniger
powder, non-animal origin, suitable for cell culture, BioReagent
Synonym(s):
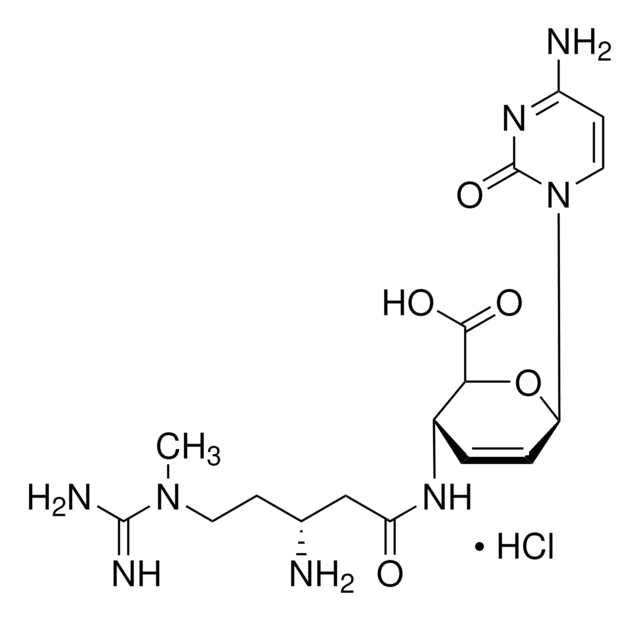
3′-[α-Amino-p-methoxyhydrocinnamamido]-3′-deoxy-N,N-dimethyladenosine dihydrochloride
About This Item
Recommended Products
product name
Puromycin dihydrochloride from Streptomyces alboniger, powder, BioReagent, suitable for cell culture
biological source
Streptomyces alboniger
Quality Level
product line
BioReagent
Assay
≥98% (HPLC)
form
powder
mol wt
544.43 g/mol
packaging
pkg of 10 mg
pkg of 100 mg
pkg of 25 mg
greener alternative product score
old score: 88
new score: 79
Find out more about DOZN™ Scoring
greener alternative product characteristics
Atom Economy
Design for Energy Efficiency
Use of Renewable Feedstocks
Design for Degradation
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
technique(s)
cell culture | mammalian: suitable
color
yellow-white
UniProt accession no.
antibiotic activity spectrum
Gram-positive bacteria
neoplastics
parasites
application(s)
agriculture
greener alternative category
Mode of action
protein synthesis | interferes
storage temp.
−20°C
SMILES string
Cl.Cl.COc1ccc(C[C@H](N)C(=O)N[C@H]2[C@@H](O)[C@@H](O[C@@H]2CO)n3cnc4c(ncnc34)N(C)C)cc1
InChI
1S/C22H29N7O5.2ClH/c1-28(2)19-17-20(25-10-24-19)29(11-26-17)22-18(31)16(15(9-30)34-22)27-21(32)14(23)8-12-4-6-13(33-3)7-5-12;;/h4-7,10-11,14-16,18,22,30-31H,8-9,23H2,1-3H3,(H,27,32);2*1H/t14-,15+,16+,18+,22+;;/m0../s1
InChI key
MKSVFGKWZLUTTO-FZFAUISWSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- in the preparation of puromycin stock solution for puromycin sensitivity assay.
- to select lentivirus-infected cells containing puromycin resistant selection marker.
Biochem/physiol Actions
Mode of Resistance: Puromycin acetyltransferase is an effective resistance gene.
Antimicrobial Spectrum: This product is active against gram-positive microorganisms, less active against acid-fast bacilli and more weakly active against gram-negative microorganisms. Puromycin can prevent growth of bacteria, protozoa, algae and mammalian cells and acts quickly, killing 99% of cells within 2 days.
Caution
Preparation Note
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
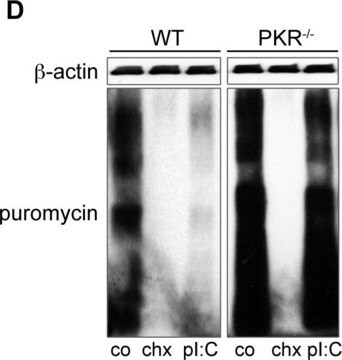
Antibiotic kill curve is a dose response experiment in which mammalian cells are subjected to increasing amounts of selection antibiotic
Related Content
Cell Culture Antibiotic Selection Guide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service