H43415
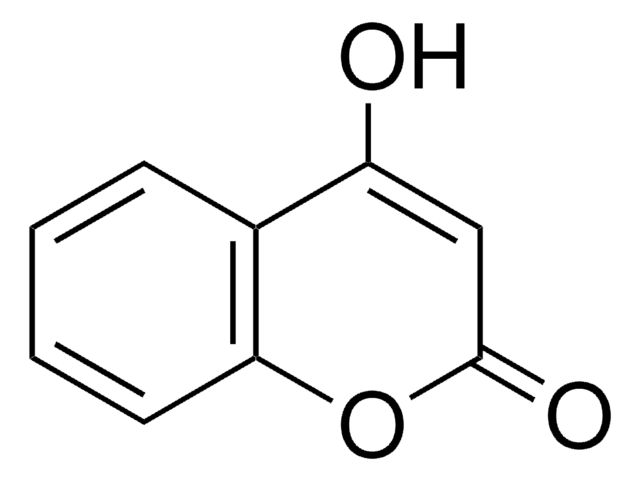
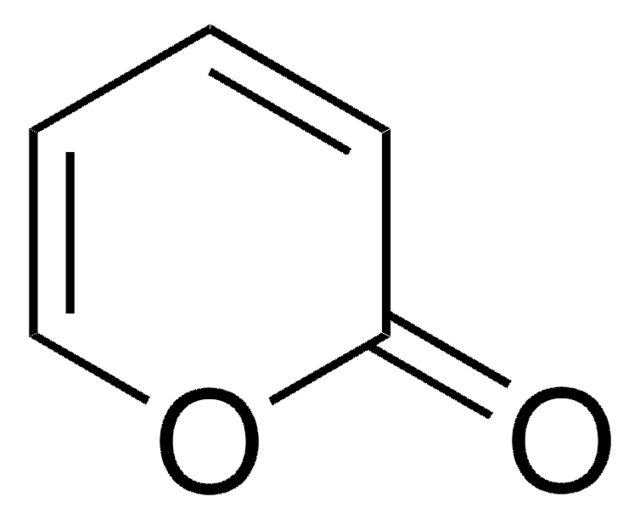
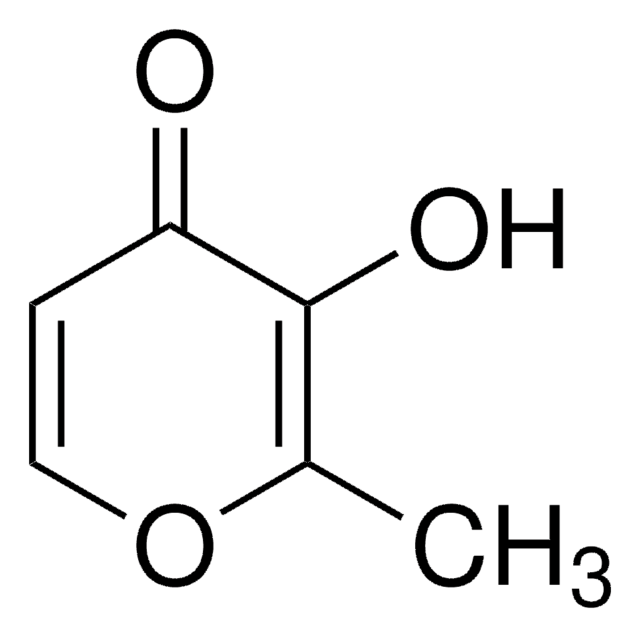
4-Hydroxy-6-methyl-2-pyrone
98%
Synonym(s):
3,5-Dihydroxysorbic acid δ-lactone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6O3
CAS Number:
Molecular Weight:
126.11
Beilstein:
113815
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
188-190 °C (dec.) (lit.)
SMILES string
CC1=CC(O)=CC(=O)O1
InChI
1S/C6H6O3/c1-4-2-5(7)3-6(8)9-4/h2-3,7H,1H3
InChI key
NSYSSMYQPLSPOD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chiho Taguchi et al.
Acta crystallographica. Section F, Structural biology and crystallization communications, 64(Pt 3), 217-220 (2008-03-08)
Polyketide synthase-1 (PKS-1) is a novel type III polyketide synthase that catalyzes the biosynthesis of hexanoyl triacetic acid lactone in Cannabis sativa (Mexican strain). PKS-1 was overproduced in Escherichia coli, purified and finally crystallized in two different space groups. The
F Kurosaki
Archives of biochemistry and biophysics, 328(1), 213-217 (1996-04-01)
6-Hydroxymellein synthase is a polyketide biosynthetic enzyme induced in carrot cells which is organized as a homodimer composed of multifunctional subunits. The synthase liberates triacetic acid lactone, instead of 6-hydroxymellein, as a derailment product when the keto-reducing reaction at the
Wenjuan Zha et al.
Journal of the American Chemical Society, 126(14), 4534-4535 (2004-04-09)
Metabolic pathway engineering is a powerful tool to synthesize structurally diverse and complex chemicals via genetic manipulation of multistep catalytic systems involved in cell metabolism. Here, we report the rational design of a fatty acid biosynthetic pathway, Brevibacterium ammoniagenes fatty
J B Spencer et al.
The Biochemical journal, 288 ( Pt 3), 839-846 (1992-12-15)
6-Methylsalicylic acid synthase has been isolated in homogeneous form from Penicillium patulum grown in liquid culture from a spore inoculum. The enzyme is highly susceptible to proteolytic degradation in vivo and in vitro, but may be stabilized during purification by
Synthesis of acetoacetyl-CoA by bovine mammary fatty acid synthase.
S Ghayourmanesh et al.
FEBS letters, 132(2), 231-234 (1981-09-28)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Benzo[h]quinoline 97%](/deepweb/assets/sigmaaldrich/product/structures/344/715/928932d2-4ca4-4402-b56c-85a80100ce17/640/928932d2-4ca4-4402-b56c-85a80100ce17.png)