All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H9N3
CAS Number:
Molecular Weight:
147.18
MDL number:
UNSPSC Code:
12352100
eCl@ss:
32151902
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
SMILES string
Cn1c(N)nc2ccccc12
InChI
1S/C8H9N3/c1-11-7-5-3-2-4-6(7)10-8(11)9/h2-5H,1H3,(H2,9,10)
InChI key
XDFZKQJLNGNJAN-UHFFFAOYSA-N
General description
The carbonyl-scavenging ability of 2-amino-1-methylbenzimidazole has been investigated. Mechanism of Menshutkin reaction between 2-amino-1-methylbenzimidazole and iodomethane has been studied in gas phase and in liquid acetonitrile. It is reported to form adducts with natural allyl, phenethyl, and benzyl isothiocyanates.
Application
2-Amino-1-methylbenzimidazole may be used for the preparation of 2- or 3-carboxy-4H-pyrimido[2,1-b]-benzazol-4-ones and novel functionalized spiropyran′s derivatives of 2H-1,3-benzoxazinone series.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
André Melo et al.
The journal of physical chemistry. B, 110(4), 1877-1888 (2006-02-14)
The quaternization reaction between 2-amino-1-methylbenzimidazole and iodomethane was investigated in the gas phase and in liquid acetonitrile. Both experimental and theoretical techniques were used in this study. In the experimental part of this work, accurate second-order rate constants were obtained
Synthesis and antiallergic activity of some acidic derivatives of 4H-pyrimido[2,1-b]benzazol-4-ones.
J J Wade et al.
Journal of medicinal chemistry, 26(4), 608-611 (1983-04-01)
Reactions of 2-aminobenzothiazole, 2-aminobenzoxazole, and 2-amino-1-methylbenzimidazole with dimethyl aminofumarate (DMAF) or diethyl ethoxymethylenemalonate (DEEM) led to 2- or 3-carboxy-4H-pyrimido[2,1-b]-benzazol-4-ones, respectively. Subsequent derivatization of these carboxylic acids gave the corresponding tetrazolylcarboxamides and tetrazoles. These acidic compounds were tested in the rat
Synthesis and structural characterization of novel 2-benzimidazolylthioureas: adducts of natural isothiocyanates and 2-amino-1-methylbenzimidazole.
Smiechowska A, et al.
Structural Chemistry, 21(5), 955-964 (2010)
Antony O Bulanov et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 71(3), 1146-1152 (2008-06-14)
Six novel functionalized spiropyran's derivatives of 2H-1,3-benzoxazinone series were synthesized by introducing the substituents with chelating ability into 2H-chromene part of the 8'-formyl-7'-hydroxy-3-methyl-4-oxo-3,4-dihydro-2H-1,3-benzoxazine-2-spiro-2'-[2H]-chromene (I) by condensation with 2-aminophenol, 2-amino-4-methylphenol, 2-amino-4-nitrophenol, 2-amino-1-methylbenzimidazole, 4-amino-4H-1,2,4-triazole, N-(4-aminophenyl)acetamide. (1)H NMR, UV/vis, IR spectroscopy combined with
Francisco J Hidalgo et al.
Journal of agricultural and food chemistry, 62(49), 12045-12051 (2014-11-25)
The carbonyl-scavenging ability of 2-amino-1-methylbenzimidazole (AMBI) and the heterocyclic aromatic amine 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) was investigated in an attempt to identify new routes that can modify the carbonyl content of foods. The reaction of both AMBI and PhIP with 2-alkenals, 2,4-alkadienals
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 412546-1G | 4061826663202 |
| 412546-5G | 4061832075952 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service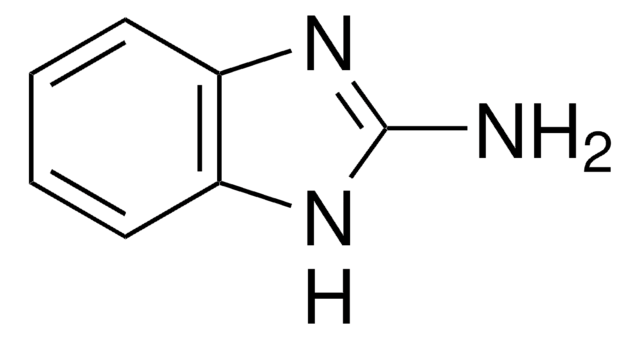


![1′,3′-Dihydro-1′,3′,3′-trimethyl-6-nitrospiro[2H-1-benzopyran-2,2′-(2H)-indole] 98%](/deepweb/assets/sigmaaldrich/product/structures/503/745/147ecd2c-44b9-46e9-a8c9-bff9a2577218/640/147ecd2c-44b9-46e9-a8c9-bff9a2577218.png)