SRP0407
Histone H2b full length human
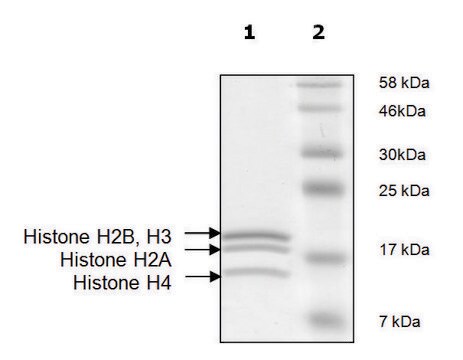
recombinant, expressed in E. coli, ≥65% (SDS-PAGE)
Synonym(s):
H2BFA, histone cluster 1, H2bg
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
biological source
human
recombinant
expressed in E. coli
Assay
≥65% (SDS-PAGE)
form
aqueous solution
mol wt
14.6 kDa
packaging
pkg of 1 mg
NCBI accession no.
UniProt accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... HIST1H2BG(8339)
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Comprehensive View of the Epigenetic Landscape Part I: DNA Methylation, Passive and Active DNA Demethylation Pathways and Histone Variants
Anna Sadakierska-Chudy
Neurotoxicity Research, 27 (2015)
Histone H2B ubiquitylation and deubiquitylation in genomic regulation.
N C T Emre et al.
Cold Spring Harbor symposia on quantitative biology, 69, 289-299 (2005-08-25)
Abba Kastin
Handbook of Biologically Active Peptides (2013)
Mary Ann Osley
Briefings in functional genomics & proteomics, 5(3), 179-189 (2006-06-15)
Histone ubiquitylation has emerged as an important chromatin modification with roles in transcription and trans-histone methylation. In the past several years, there has been dramatic progress in the identification of factors that control ubiquitin attachment to the core histones H2A
John J Wyrick et al.
Biochimica et biophysica acta, 1789(1), 37-44 (2008-08-05)
In eukaryotic cells, the genome is packaged with histones H2A, H2B, H3, and H4 to form nucleosomes. Each of the histone proteins is extensively post-translationally modified, particularly in the flexible N-terminal histone tail domains. Curiously, while post-translational modifications in histone
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service