SRP0408
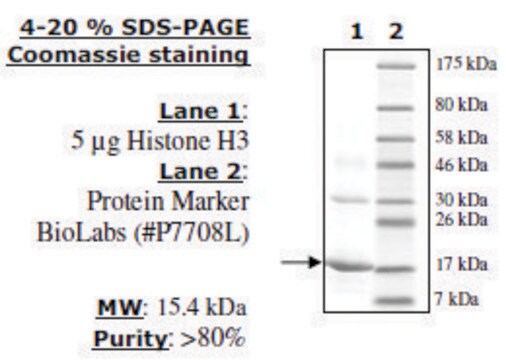
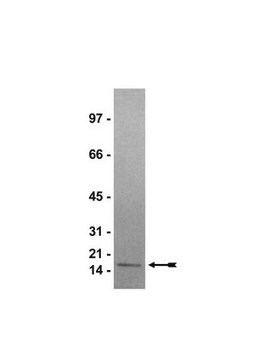
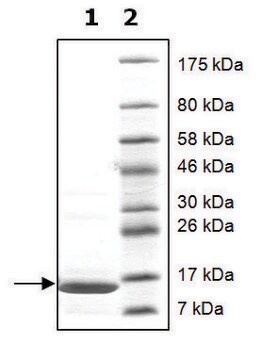
Histone Octamer full length human
recombinant, expressed in E. coli, ≥90% (SDS-PAGE)
Synonym(s):
Histone Octamer
About This Item
Recommended Products
biological source
human
recombinant
expressed in E. coli
Assay
≥90% (SDS-PAGE)
form
aqueous solution
mol wt
113.8 kDa
packaging
pkg of 100 μg
technique(s)
cell based assay: suitable
solubility
water: soluble
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... HIST1H2BG(8339) , HIST2H2AC(8338) , HIST3H3(8290) , HIST4H4(121504)
General description
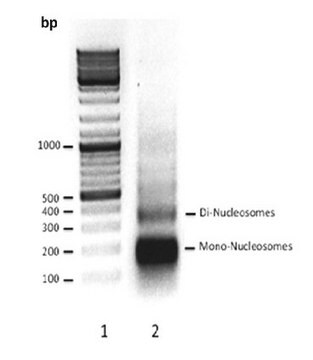
The histone octamer is a versatile protein assembly that has evolved to serve two opposing functions within the cell. It is required to bind and bend DNA to achieve fivefold compaction and partial charge neutralization of DNA, while also needing to release specific segments of DNA in a coordinated manner to allow the access of DNA-processing enzymes at the appropriate time. A modular assembly of histone dimers (consisting of either H2A and H2B or H3 and H4) binds to approximately 30 bp of DNA and is connected in a flexible yet stable manner to form a fundamental superhelical ′ramp′ with evenly spaced DNA-binding platforms.
Application
Biochem/physiol Actions
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service