158534
Trifluoromethanesulfonic acid
reagent grade, 98%
Synonym(s):
TFMSA, Triflic acid
About This Item
Recommended Products
grade
reagent grade
Quality Level
vapor density
5.2 (vs air)
vapor pressure
8 mmHg ( 25 °C)
Assay
98%
form
liquid
refractive index
n20/D 1.327 (lit.)
bp
162 °C (lit.)
density
1.696 g/mL at 25 °C (lit.)
SMILES string
OS(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S/c2-1(3,4)8(5,6)7/h(H,5,6,7)
InChI key
ITMCEJHCFYSIIV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Friedel-Crafts acylation of aromatic compounds with methyl benzoate.
- Addition reaction of dialkyl disulfides to terminal alkynes.
- Synthesis of a single cyclic tetrasiloxane containing propylammonium trifluoromethanesulfonate and methyl side-chain groups (Am-CyTS).
- Preparation of starting reagents for the synthesis of fluorinated 2,5-substituted 1-ethyl-1H-benzimidazole derivatives.
- Synthesis of aryl triflates, the lactonization of alkenoic acids, and the formation of E-alkenes.
accessory
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Met. Corr. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
>332.1 °F - Pensky-Martens closed cup
Flash Point(C)
> 166.7 °C - Pensky-Martens closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
Protocols
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service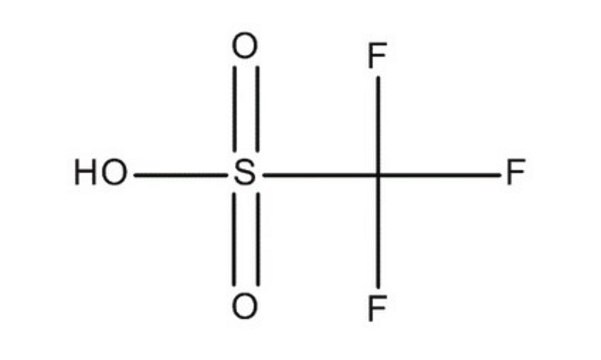




![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)