176176
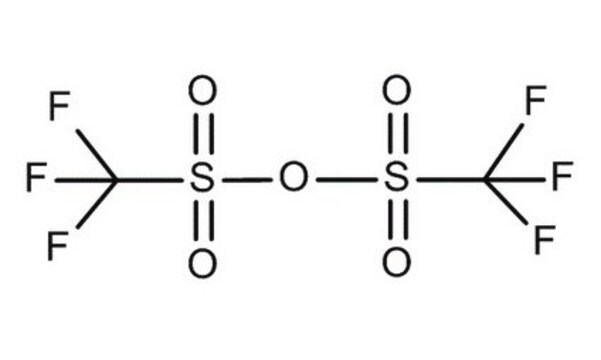
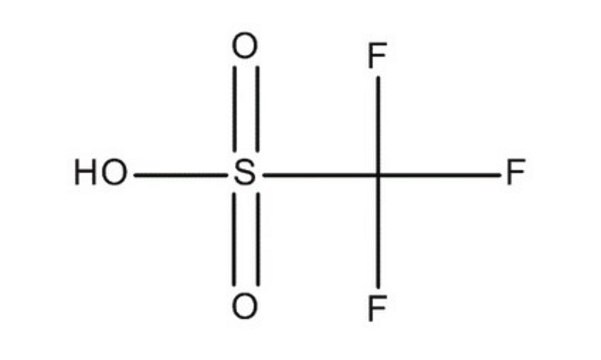
Trifluoromethanesulfonic anhydride
99%
Synonym(s):
Triflic anhydride
About This Item
Recommended Products
vapor density
5.2 (vs air)
Quality Level
vapor pressure
8 mmHg ( 20 °C)
Assay
99%
form
liquid
refractive index
n20/D 1.321 (lit.)
bp
81-83 °C (lit.)
density
1.677 g/mL at 25 °C (lit.)
functional group
fluoro
triflate
SMILES string
FC(F)(F)S(=O)(=O)OS(=O)(=O)C(F)(F)F
InChI
1S/C2F6O5S2/c3-1(4,5)14(9,10)13-15(11,12)2(6,7)8
InChI key
WJKHJLXJJJATHN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Reagent for stereoselective synthesis of mannosazide methyl uronate donors
Activator for direct glycosylation with anomeric hydroxy sugars
- A Reactant in the synthesis of Dipentaerythritol hexatriflate via triflatation method and Azido-diphenyl-acetic acid.
- Catalyst for glycosylation for synthesis of polysaccharides.
- Reagent for stereoselective synthesis of mannosazide methyl uronate donors.
- Activator for direct glycosylation with anomeric hydroxy sugars
- A methylation reagent to synthesize trifluoromethylated compounds by direct introduction of CF3 group to (hetero)arenes.
- A reagent to prepare substituted tetrazoles from secondary amides using sodium azide.
- A reagent in Bischler−Napieraiski cyclization reaction along with 4-(N,N-dimethylamnino)pyridine.
accessory
recommended
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
not determinedboils before flash
Flash Point(C)
not determinedboils before flash
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 176176-250G | 4061838752789 |
| 176176-4KG | 4061833688458 |
| 176176-500G | 4061833544822 |
| 176176-10G | 4061838752765 |
| 176176-1KG | 4061838752772 |
| 176176-50G | 4061838752796 |
| 176176-5G | 4061838752802 |
| 176176-PZ | 4061823818766 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service