296678
(S)-(−)-1,2,4-Butanetriol
98%
Synonym(s):
(2S)-(-)-Butane-1,2,4-triol, (S)-(-)-1,2,4-Trihydroxybutane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOCH2CH2CH(OH)CH2OH
CAS Number:
Molecular Weight:
106.12
Beilstein:
1719408
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
optical activity
[α]19/D −28±2, c = 1 in methanol
optical purity
ee: 99% (GLC)
refractive index
n20/D 1.475 (lit.)
bp
150 °C/0.04 mmHg (lit.)
density
1.19 g/mL at 25 °C (lit.)
SMILES string
OCC[C@H](O)CO
InChI
1S/C4H10O3/c5-2-1-4(7)3-6/h4-7H,1-3H2/t4-/m0/s1
InChI key
ARXKVVRQIIOZGF-BYPYZUCNSA-N
Looking for similar products? Visit Product Comparison Guide
General description
(S)-(-)-1,2,4-Butanetriol can be prepared via reduction of (S)-malic acid in the presence of borane-dimethyl sulfide.
Application
(S)-(-)-1,2,4-Butanetriol may be used as a starting material in the enantioselective total syntheses of (+)-azimine and (+)-carpaine.
It can also be used to prepare the following organic building blocks:
It can also be used to prepare the following organic building blocks:
- (+)-3,4-epoxy-1-butanol
- (2S,4S)-4-(hydroxymethyl)-2-ferrocenyl-1,3-dioxane
- (S)-1,2,4-triacetoxybutane via acetylation with acetic anhydride
- (S)-1,2,4-tris-(3,5-dinitrobenzoy1oxy)butane via esterification with 3,5-dinitrobenzoyl chloride
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Facile access to (S)-1, 2, 4-butanetriol and its derivatives.
Hanessian S, et al.
Canadian Journal of Chemistry, 62(11), 2146-2147 (1984)
Asymmetric Michael addition of thiophenol to maleic acid esters
Yamashita H and Mukaiyama T
Chemistry Letters (Jpn), 14(3), 363-366 (1985)
Enantioselective total synthesis of (+)-azimine and (+)-carpaine.
Sato T, et al.
Organic Letters, 5(21), 3839-3842 (2003)
An efficient asymmetric synthesis of 2-substituted ferrocenecarboxaldehydes
Riant O, et al
The Journal of Organic Chemistry, 62(20), 6733-6745 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service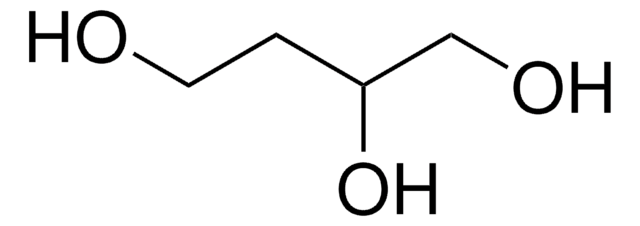



![Poly[(R)-3-hydroxybutyric acid] natural origin](/deepweb/assets/sigmaaldrich/product/structures/129/476/7d1c924b-f644-4889-a2d6-d7a923ce382c/640/7d1c924b-f644-4889-a2d6-d7a923ce382c.png)