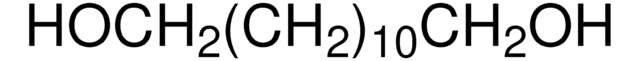T66206
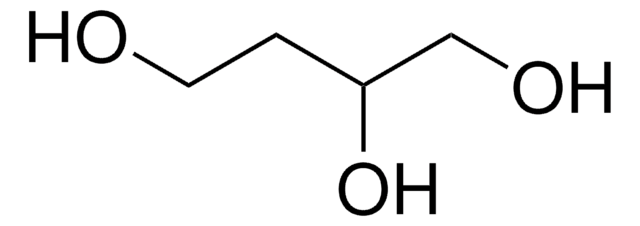
1,2,6-Hexanetriol
96%
Synonym(s):
1,2,6-Trihydroxyhexane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HO(CH2)4CH(OH)CH2OH
CAS Number:
Molecular Weight:
134.17
Beilstein:
1304479
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
4.63 (vs air)
Quality Level
vapor pressure
<0.01 mmHg ( 20 °C)
Assay
96%
refractive index
n20/D 1.476 (lit.)
bp
178 °C/5 mmHg (lit.)
density
1.109 g/mL at 25 °C (lit.)
SMILES string
OCCCCC(O)CO
InChI
1S/C6H14O3/c7-4-2-1-3-6(9)5-8/h6-9H,1-5H2
InChI key
ZWVMLYRJXORSEP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1,2,6-Hexanetriol has been used as a precursor to synthesize a trivalent linker containing three thiol moieties (triskelion) for the regioselective terminal activation of chitosan.
It can be used to prepare:
It can be used to prepare:
- 1,6-Hexanediol (key polymer precursor to polyesters) via hydrodeoxygenation process in the presence of Rh–ReOx/SiO2 catalyst.
- Tetrahydropyran-2-methanol, a six-membered oxide ring, by reacting with ethylene carbonate.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
383.0 °F - closed cup
Flash Point(C)
195 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
From 5-hydroxymethylfurfural (HMF) to polymer precursors: catalyst screening studies on the conversion of 1, 2, 6-hexanetriol to 1, 6-hexanediol
Buntara T, et al.
Topics in Catalysis, 55(7-10), 612-619 (2012)
Cyclic ethers made by pyrolysis of carbonate esters
Pattison DB
Journal of the American Chemical Society, 79(13), 3455-3456 (1957)
Regioselective chitosan end-group activation: the triskelion approach
Pickenhahn VD, et al.
Royal Society of Chemistry Advances, 7(30), 18628-18638 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service