In Vitro Differentiation of Human iPS Cells Into Colon Organoids in Serum-Free Cell Culture Conditions
Kevin Su, Nick Asbrock, Vic Chu, Ph.D., Stefanie Hoffmann, M.S., Philip Hewitt, Ph.D
Organoids are complex self-organized 3D cell culture models that are often derived from stem cells.1 Organoids have been generated from diverse tissues including brain,2 intestine,3 stomach,4 colon,5 liver,6 pancreas,6 lung,7 kidney8 and patient-derived tumors.9 Epithelial intestinal organoids, often referred to as enteroids or mini-guts, maintain the physiological characteristics of the gastrointestinal system and have been a useful cell culture tool to model intestinal development and disease including the study of colon cancer, celiac disease, inflammatory bowel diseases and host microbiome interactions.10 Traditional isolation techniques developed by Clevers et al.1 rely on lengthy primary tissue isolation, either from mouse or from difficult-to-source human tissue samples. Induced pluripotent stem cell-derived organoids would enable the rapid generation of patient-specific cell models from a wider range of human donors. We have generated a human iPSC-derived colon organoid system that provides highly characterized, assay-ready cryopreserved human colonic organoids and expansion media. Furthermore, our optimized serum-free media and reagents can be used to derive colonic organoids from any human iPS cell line using a simple three-step differentiation process.

Figure 1.Human colonic organoid differentiation workflow. Human colonic organoids can be generated from human iPS cells using a three-step differentiation protocol through definitive endoderm, hindgut endoderm, and colonic organoid expansion stages.
SCM302: Definitive Endoderm Induction Medium, SCM303: Hindgut Induction Medium, SCM304: 3dGRO™ Human Colon Organoid Expansion Medium
Organoid Culture Protocols
Step 1: Differentiation of Human iPS Cells to Definitive Endoderm (Day 0-4)
Note: Start with high-quality undifferentiated human ES/iPS cells (SCC271) that are ~70-80% confluent and contain <5% differentiated cells. The following protocol is for differentiation of one well of a six-well tissue-culture treated plate. Indicated volumes are for a single well. Adjust volumes as necessary.
- Prepare single-cell passaging media. Add ROCK inhibitor (ROCKi) Y-27632 (SCM075) to 7-10 mL Human ES/iPS Expansion Media (SCM130) to a final concentration of 10 µM.
- Coat a 6-well plate with ECM Gel (CC131-5ML).
- Aspirate the medium. Wash the well with 2 mL of DMEM/F12 or 1X PBS. Aspirate and add 1 mL of Accumax™ (A7089) to the well. Incubate for 5-6 minutes at 37̣ °C. Tap the plate firmly against the palm of your hand to help dislodge the cells.
- Add 1 mL of the single-cell passaging media (from step 1) to the well. Pipette up and down 1-3 times with a 5 mL pipettor to dislodge the cells. Be careful to not introduce any bubbles.
- Collect the dissociated cells in a 15 mL conical tube. Add 1 mL single-cell passaging media to the well and collect any remaining cells for transfer to the 15 mL conical tube containing the cell suspension. Centrifuge the tube at 140 x g for 5 minutes and aspirate the supernatant.
- Resuspend the cell pellet in 1 mL of single-cell passaging media. Count the total number of live cells using Trypan blue (T8154) and a hemocytometer.
- Add 1x106 cells per well to an ECM Gel (CC131-5ML) -coated 6-well plate. The media used should be the single-cell passaging media (from step 1); Total volume = 3 mL per well. Incubate at 37 °C overnight.
- Aspirate the media from the well. Add 2 mL of Definitive Endoderm Induction Medium (SCM302) to the well and incubate at 37 °C overnight.
- Repeat step 8 for day 2 and day 3. Analyze cells by flow cytometry on day 4. Before proceeding to step 2, cells should be >85% positive for the endoderm markers CXCR4, c-Kit, Sox-17, and FOXA2, and negative for PDGFR.
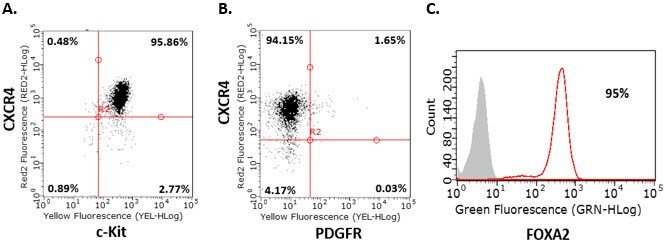
Figure 2.Endoderm differentiation of human iPS cells. Flow cytometry analysis of human iPSC-derived definitive endoderm cells for endoderm markers demonstrates that cells are CXCR4+, c-Kit+, Sox-17+ , PDGFR- and FOXA2+ after four days of differentiation.
Step 2: Hindgut Endoderm Differentiation (Day 4-8)
- Remove a 6-well plate containing day 4 definitive endoderm cultures from the incubator.
- Aspirate the media from each well.
- Wash each well with 3 mL 1X PBS.
- Add 2 mL of the pre-warmed Hindgut Induction Media (SCM303) into each well.
- Immediately transfer plate into a 37 °C incubator with 5% CO2 for 24 hours.
- Repeat steps 2-5 for a total of 5 days.
- Day 5 of hindgut endoderm differentiation is a critical timepoint to determine the expression of CDX2. Expression of CDX2 must be >60% to proceed to step 3. If expression of CDX2 is lower than 60%, carry on an additional day of HE differentiation and re-check for CDX2 expression.

Figure 3.Differentiation of definitive endoderm cells into hindgut endoderm cells. Morphology of hindgut endoderm cells at A) day 2 B) day 3 and C) day 4 post hindgut endoderm induction.
Step 3: Expansion and Cryopreservation of Colonic Organoids (Day 8-48)
Expansion of Colon Organoids
- Remove a 6-well plate containing day 8 hindgut endoderm cultures from the incubator.
- Aspirate the media from each well.
- Wash each well with 3 mL of 1X PBS.
- Add 2 mL of the pre-warmed 3dGRO™ Human Colon Organoid Expansion Media (SCM304) into each well.
- Immediately transfer plate into a 37 °C incubator with 5% CO2 for 48 hours.
- Replace media every 2 days with 3dGRO™ Human Colon Organoid Expansion Media (SCM304) until Day 12 of differentiation.
Note: Colon spheroids will form at the end of Day 12, which will be embedded in Matrigel® matrix using the 3dGRO™ Human Colon Organoid Expansion Media (SCM304) to generate mature colonic organoids.
- Add ROCKi Y-27632 (SCM075) at a final concentration of 10 µM to the Human Colon Organoid Expansion Media (SCM304).
- Thaw a sufficient volume of Growth Factor Reduced Matrigel at 4 °C overnight or on ice.
- On Day 12 of colonic epithelial cell differentiation, remove cells from the incubator and collect spheroids floating in the media to a 15 mL conical tube.
- Wash each well with 3 mL 1X PBS.
- Detach monolayers containing spheroids (non-floating spheroids) with 1 mL of Accumax (A7089) and incubate at 37 °C for 5-10 minutes.
- Once the monolayer is loosened, gently pipette cells 4-5 times with a P-1000 pipette and transfer the 1 mL cell suspension into the conical tube prepared in step 9 above.
- Centrifuge at 265 x g at 4 °C for 5 minutes.
- Aspirate the supernatant and wash once with 4 mL of 1X PBS.
- Centrifuge at 265 x g at 4 °C for 5 minutes and aspirate the supernatant.
- Gently flick the bottom of the conical tube to dislodge the pellet.
- Place Matrigel® matrix in the TC hood and quickly remove 1 mL and add it to the dislodged cell pellet from step 16.
- Resuspend the cell pellet gently in Matrigel® matrix with a P-1000 pipette set at 900 µL. Avoid introducing air bubbles during the resuspension.
- Immediately place cell suspension on ice for 5 minutes to prevent gelling.
- Dilute the 1 mL Matrigel® matrix /cell suspension 1:50-1:100 in fresh Matrigel® matrix and seed 50 µL/dome in each well of a 24-well plate (for example: for a 1:100 dilution, combine 10 µL of the matrix/cells from step 18 with 40 µL of fresh matrix. Plate 50 µL/well). See figure 4 below.
- Immediately incubate the 24-well plate at 37 °C for 10 minutes.
- Once the domes are gelled, add 1 mL of 3dGRO™ Human Colon Organoid Expansion Media (SCM304)+ 10 µM ROCKi (SCM075).
- Media can be refreshed every two days with 1 mL fresh 3dGRO™ Human Colon Organoid Expansion Media (SCM304).
- Organoids can be passaged every 10-12 days using the 3dGRO™ Organoid Dissociation Reagent (SCM300) at a split ratio of 1:3 to 1:4.

Figure 4.Human colon organoids. A) Colonic organoids encapsulated in Matrigel® matrix domes, two days post-thaw. B) By day 10-12 in culture, colon organoids occupy 85-90% of the dome, and are ready to be passaged.
Cryopreservation of Colon Organoids
Human colonic organoids can be cryopreserved using the 3dGRO™ Organoid Freezing Media (SCM301). This protocol is based on not using any dissociation reagents during passaging of human colonic organoids for cryopreservation. The recommended starting number of domes per vial is four per vial, with the assumption that the density of each dome is at 90%. If the density is less than 90%, freeze down more domes per vial.
- When organoids are ready to be passaged for cryopreservation, remove the plate from the incubator.
- Resuspend each Matrigel matrix dome in its existing 1 mL of media by pipetting up and down 5 times with a P-1000 pipette.
- Transfer organoid suspension into a conical tube.
- Continue to disperse organoids by pipetting with a 10 mL pipette along the side of the wall of the conical tube 10 times.
- Centrifuge the conical tube at 265 x g to 700 x g.
- Aspirate the supernatant and resuspend pellet with 1 mL of 1X PBS.
- Continue to disperse organoid pellet with a P-1000 pipette by pipetting up and down 20 times.
- Add 5 mL of 1X PBS to the organoid suspension and centrifuge at 700 x g for 5 minutes at 4 °C. Centrifuge for an additional 5 minutes if residual organoid fragments are present in the supernatant.
- Aspirate the supernatant and resuspend pellet with 1 mL of 3dGRO™ Organoid Freezing Media (SCM301).
- Resuspend the pellet 5 times with a P-1000 pipette and add the rest of the freezing media needed for four domes per vial in 1 mL.
- Transfer the cells in the cryopreservation vials to a freezing container (such as a Mr. Frosty) and place in a -80 °C freezer for 24-72 hours.
- Transfer cryopreserved human colonic organoids into a liquid nitrogen tank for long-term storage.
Results

Figure 5.Morphology of human iPS cell-derived colonic organoids. Mature human colon organoids have complex morphologies when cultured in three dimensions. A) 4X magnification B) 10X magnification.
Human Colon Organoid Characterization |
|---|
CDX2
CA-IV/DAPI
Mucin-5B/DAPI
Mucin-2/F-Actin/DAPI

CDX2

CA-II/DAPI

CA-IV/DAPI

Mucin-5B/DAPI

Mucin-2/F-Actin/DAPI

E-Cad/DAPI
Figure 6. Immunocytochemical (ICC) characterization of human colon organoids. Human iPS cell-derived colonic organoids are positive for CDX2, α-carbonic anhydrase-II, α-carbonic anhydrase-IV, mucin-5B, mucin-2 and E-cadherin.

Figure 7.Immunohistochemical (IHC) characterization of human colon organoids. A) Goblet cells identified with alcian blue staining B) proliferating cells identified using Ki67 antibody (red) C) nuclei and plasma protein identified using H&E staining.
Conclusions
We have developed a robust three-step differentiation protocol to generate human colonic organoids from human induced pluripotent stem cells (iPSCs). Colon organoids generated using this protocol express mature colon markers CDX2, α-carbonic anhydrase-II, α-carbonic anhydrase-IV, mucin 5B, mucin 2 and E-cadherin, and can be serially passaged over multiple passages without losing colon phenotypes. These organoids and serum-free media will provide researchers and drug discovery with highly validated new 3D cell models to study intestinal diseases.
References
Zaloguj się lub utwórz konto, aby kontynuować.
Nie masz konta użytkownika?