49291
Glucosidase from Aspergillus niger
powder, ≥750 U/g
Synonym(s):
Cellobiase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
powder
specific activity
≥750 U/g
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
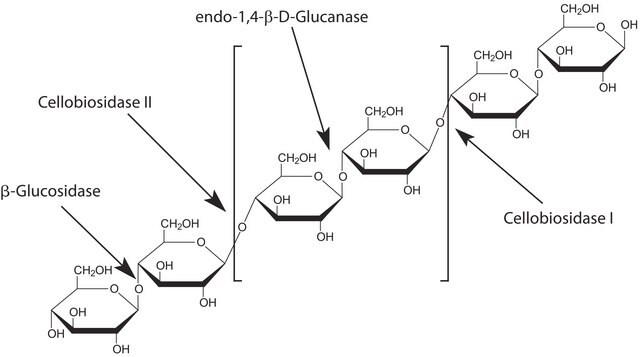
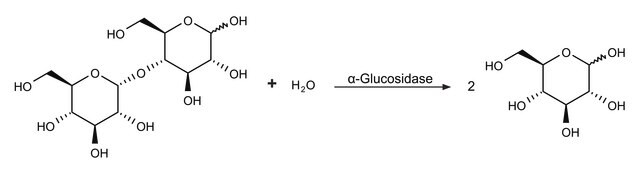
Glucosidase catalyzes the hydrolysis of α-1,4 linkages with a substrate preference for maltose, maltotriose and maltotetraose. Reactivity with large polysaccharides like dextrin and starch have also been described.
Glucosidase catalyzes the hydrolysis of α-1,4 linkages with a substrate preference for maltose, maltotriose and maltotetraose. Reactivity with large polysaccharides like dextrin and starch have also been described.
Unit Definition
1 U corresponds to the amount of enzyme which hydrolyzes 1 μmol p-nitrophenyl-β-D-glucopyranoside per minute at pH 4.0 and 37 °C; may contain α- and β-glucosidase
Other Notes
Characterization; Ethanol production from paper-mill waste fibre
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T Watanabe et al.
European journal of biochemistry, 209(2), 651-659 (1992-10-15)
Beta-glucosidase was purified from a crude cellulase preparation from Aspergillus niger by affinity chromatography on a methacrylamide-N-methylene-bis-methacrylamide copolymer bearing cellobiamine. The purified enzyme was a dimer with an isoelectric point of 4.0. The molecular mass of the enzyme was estimated
McCleary, B.V., and Harrington, J.,
Methods in Enzymology, 160, 575-575 (1988)
Action of α-D-glucosidase from Aspergillus niger towards dextrin and starch
M. Ota et al
Carbohydrate Polymers, 78, 287-291 (2009)
Substrate specificity and subsite affinities of crystalline α-glucosidase from Aspergillus niger
A. Kita et al
Agricultural and Biological Chemistry, 55, 2327-2335 (1991)
J H Pazur et al.
Carbohydrate research, 58(1), 193-202 (1977-09-01)
The action patterns of glucoamylase (amyloglucosidase) and glucosyltransferase (transglucosylase) on D-[1-14C]glucose, [1-14C]maltose, and [1-14C]malto-oligosaccharides (labeled at position 1 of the D-glucose group at the reducing end) have been investigated by paper-chromatographic and oligosaccharide-mapping techniques. Under the conditions of the experiments
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service