B1215000
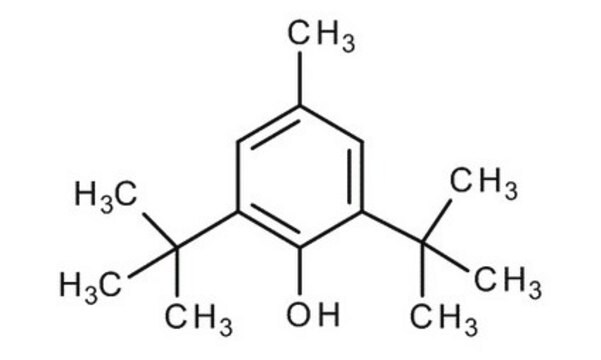
Butylhydroxytoluene
European Pharmacopoeia (EP) Reference Standard
Synonym(s):
2,6-Di-tert-butyl-4-methylphenol, 2,6-Di-tert-butyl-p-cresol, BHT, Butylated hydroxytoluene, Butylhydroxytoluene, DBPC
About This Item
Recommended Products
grade
pharmaceutical primary standard
vapor density
7.6 (vs air)
vapor pressure
<0.01 mmHg ( 20 °C)
autoignition temp.
878 °F
manufacturer/tradename
EDQM
bp
265 °C (lit.)
mp
69-73 °C (lit.)
application(s)
cleaning products
cosmetics
food and beverages
personal care
pharmaceutical
format
neat
storage temp.
2-8°C
SMILES string
Cc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C
InChI
1S/C15H24O/c1-10-8-11(14(2,3)4)13(16)12(9-10)15(5,6)7/h8-9,16H,1-7H3
InChI key
NLZUEZXRPGMBCV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Multicomponent analysis of fat- and water-soluble vitamins and auxiliary substances in multivitamin preparations by qNMR.: This study utilizes Butylhydroxytoluene (BHT) as an auxiliary substance for its antioxidant properties in multivitamin preparations. The research demonstrates the effectiveness of BHT in maintaining the oxidative stability of vitamins, ensuring their longevity and efficacy in pharmaceutical applications (Eiff et al., 2015).
- Investigation of the stabilizing effects of antioxidants and benzophenone-3 on desonide photostability.: The study explores the use of BHT as an antioxidant to enhance the photostability of desonide. Findings indicate that BHT significantly improves the stability of the compound under light exposure, making it a valuable preservative in pharmaceutical and biotechnological formulations (Rosa et al., 2014).
- Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants.: This research highlights BHT′s role as a comparative antioxidant in studying the properties of cannabinoids. BHT′s well-documented antioxidant mechanisms provide a benchmark for evaluating the efficacy of other compounds in oxidative stress research (Borges et al., 2013).
- Kinetic study of the quenching reaction of singlet oxygen by common synthetic antioxidants.: The kinetic analysis of BHT′s ability to quench singlet oxygen demonstrates its superior antioxidant capabilities compared to other synthetic antioxidants. This property is crucial for developing pharmaceutical formulations that require high oxidative stability (Kim et al., 2009).
Packaging
Other Notes
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
260.6 °F - open cup
Flash Point(C)
127 °C - open cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
HPLC Analysis of Phenolic Antioxidants on Ascentis® Express C18 2.7 μm
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service