807796
Cyrene™
BioRenewable, DMF and NMP Substitute
Synonym(s):
Dihydrolevoglucosenone
About This Item
Recommended Products
Quality Level
Assay
≥98.5% (GC)
form
liquid
greener alternative product characteristics
Designing Safer Chemicals
Safer Solvents and Auxiliaries
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
technique(s)
: 0.5% using Water (by Karl Fischer)
bp
227 °C
mp
-18 °C
density
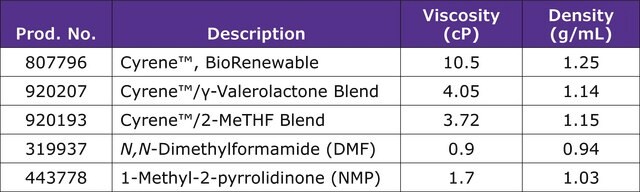
1.25 g/mL
greener alternative category
, Aligned
SMILES string
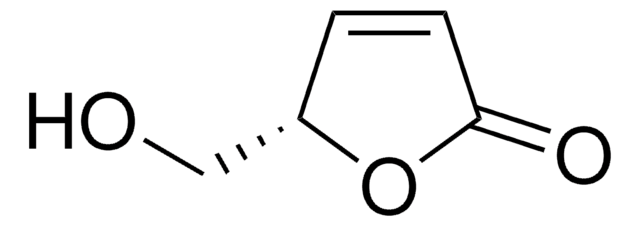
O1[C@H]2OC[C@@H]1CCC2=O
InChI
1S/C6H8O3/c7-5-2-1-4-3-8-6(5)9-4/h4,6H,1-3H2/t4-,6+/m0/s1
InChI key
WHIRALQRTSITMI-UJURSFKZSA-N
General description
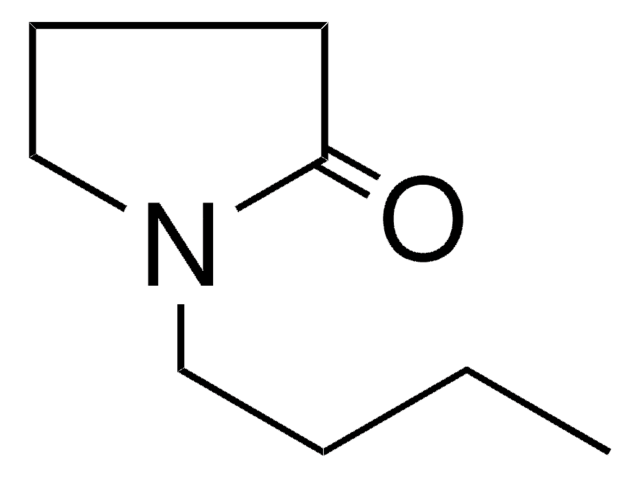
Cyrene is a biobased dipolar, safe for end-of-life disposal, decomposing into CO2 and H2O. It is an aprotic alternative to common solvents that are of environmental concern. Cyrene™ is an alternative for many solvents classified by REACH as Substances of Very High Concern (SVHC), such as N-Methylpyrrolidone (NMP) and N,N-Dimethylformamide (DMF).
Application
1. Dispersive ability for graphene solutions.
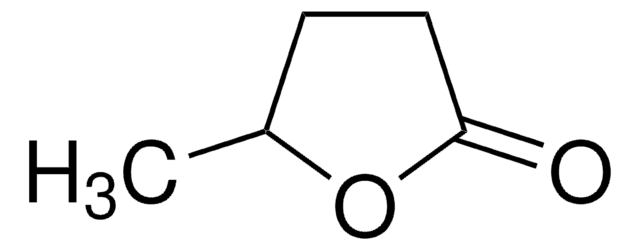
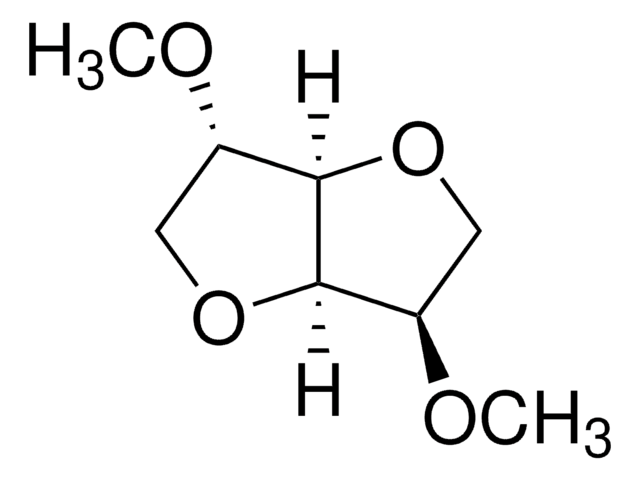
2. Alternative to DMF in the synthesis of metal-organic frameworks.
3. Organic synthesis:
- Cacchi-type annulation
- Synthesis of urea
- HATU Amide Coupling - Replacement for DMF in amide and dipeptide coupling reactions.
- Suzuki-Miyaura coupling reaction.
- Sonogashira coupling reaction.
- Reductive homocoupling reaction.
Features and Benefits
- 99% biodegradation in 28 days
- Stable during incineration
- Not mutagenic or genotoxic
- ASTM D6866 - Standard Test Methods for Determining the Biobased Content
- Made from Renewable Resource − Cellulose
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
226.4 °F - closed cup
Flash Point(C)
108 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service