692980
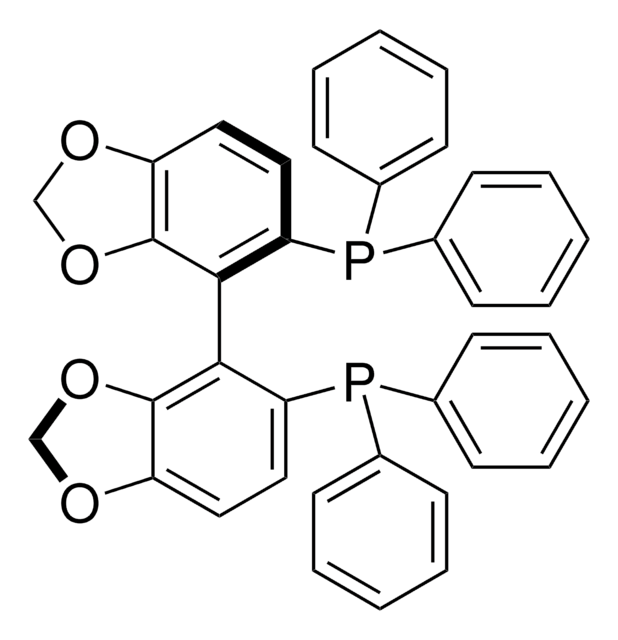
(S)-DTBM-SEGPHOS®
≥94%
Synonym(s):
(S)-(+)-5,5′-Bis[di(3,5-di-tert-butyl-4-methoxyphenyl)phosphino]-4,4′-bi-1,3-benzodioxole
About This Item
Recommended Products
Assay
≥94%
form
powder
functional group
phosphine
SMILES string
COc1c(cc(cc1C(C)(C)C)P(c2cc(c(OC)c(c2)C(C)(C)C)C(C)(C)C)c3ccc4OCOc4c3-c5c6OCOc6ccc5P(c7cc(c(OC)c(c7)C(C)(C)C)C(C)(C)C)c8cc(c(OC)c(c8)C(C)(C)C)C(C)(C)C)C(C)(C)C
InChI
1S/C74H100O8P2/c1-67(2,3)47-33-43(34-48(61(47)75-25)68(4,5)6)83(44-35-49(69(7,8)9)62(76-26)50(36-44)70(10,11)12)57-31-29-55-65(81-41-79-55)59(57)60-58(32-30-56-66(60)82-42-80-56)84(45-37-51(71(13,14)15)63(77-27)52(38-45)72(16,17)18)46-39-53(73(19,20)21)64(78-28)54(40-46)74(22,23)24/h29-40H,41-42H2,1-28H3
InChI key
ZNORAFJUESSLTM-UHFFFAOYSA-N
Application
- Enantioselective synthesis of secondary allylic alcohols via enantioselective reductions of α,β-unsaturated ketones
- Stereoselective preparation of cyclohexanone derivatives via rhodium-catalyzed rearrangement of allyl or allenic cyclobutanols
- Cu-catalyzed asymmetric conjugate reduction of beta-substituted unsaturated phosphonates to give optically active beta-stereogenic alkylphosphonates
- Asymmetric synthesis of cyclohexenones via Rh-catalyzed desymmetrization through enantioselective C-C bond activation and ring expansion of allenyl cyclobutanols
- Enantioselective synthesis and crystal structure of P-stereogenic alkynylphosphine oxides by Rh-catalyzed [2+2+2] cycloaddition
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Hydrogenation, Asymmetric Catalysis, Binap, SEGPHOS®, Aldol reaction, Alkenylation, Arylation, Mannich reaction, Fluorination, Michael addition, Hydrosilylation, Cycloaddition, Takasago
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service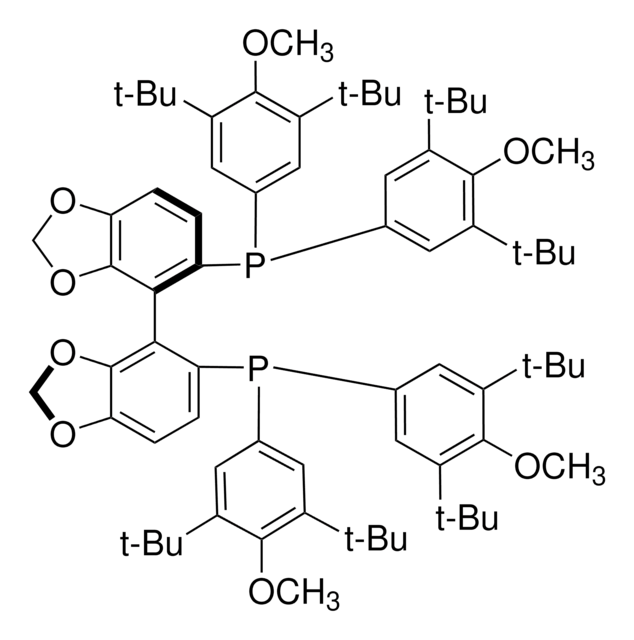


![(R)-(6,6′-Dimethoxybiphenyl-2,2′-diyl)bis[bis(3,5-dimethylphenyl)phosphine] ≥97% (31P-NMR), optical purity ee: ≥99%](/deepweb/assets/sigmaaldrich/product/structures/377/820/32e028a2-0c7d-460d-8b6f-668b6d6a1523/640/32e028a2-0c7d-460d-8b6f-668b6d6a1523.png)
![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)
![(R)-(6,6′-Dimethoxybiphenyl-2,2′-diyl)bis[bis(3,5-di-tert-butylphenyl)phosphine] ≥97%, optical purity ee: ≥99%](/deepweb/assets/sigmaaldrich/product/structures/389/033/e3c61de2-e5d4-4141-be57-0eb722850a37/640/e3c61de2-e5d4-4141-be57-0eb722850a37.png)