692395
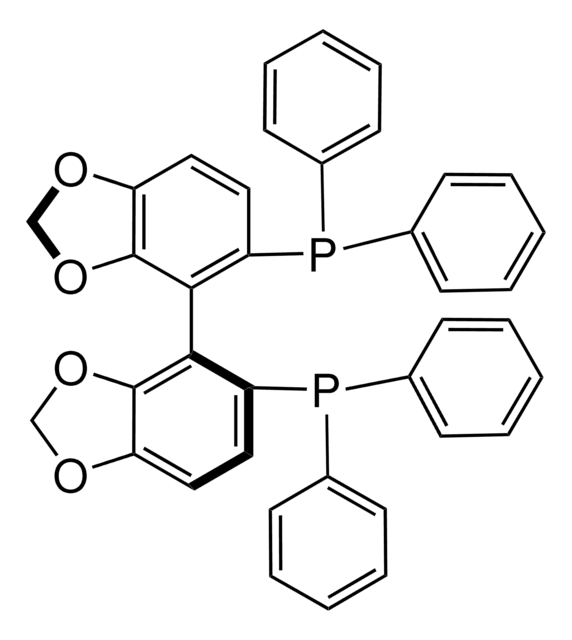
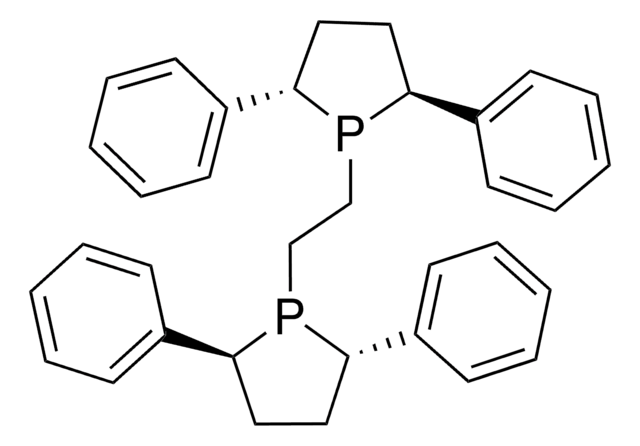
(R)-SEGPHOS®
≥94%
Synonym(s):
(R)-(+)-5,5′-Bis(diphenylphosphino)-4,4′-bi-1,3-benzodioxole, [4(R)-(4,4′-bi-1,3-benzodioxole)-5,5′-diyl]bis[diphenylphosphine]
About This Item
Recommended Products
Quality Level
Assay
≥94%
form
powder
optical activity
[α]20/D +11°, c = 0.5 in chloroform
mp
168-172 °C
functional group
phosphine
SMILES string
C1Oc2ccc(P(c3ccccc3)c4ccccc4)c(c2O1)-c5c6OCOc6ccc5P(c7ccccc7)c8ccccc8
InChI
1S/C38H28O4P2/c1-5-13-27(14-6-1)43(28-15-7-2-8-16-28)33-23-21-31-37(41-25-39-31)35(33)36-34(24-22-32-38(36)42-26-40-32)44(29-17-9-3-10-18-29)30-19-11-4-12-20-30/h1-24H,25-26H2
InChI key
RZZDRSHFIVOQAF-UHFFFAOYSA-N
Application
- Nickel-catalyzed asymmetric α-arylation and heteroarylation of ketones with chloroarenes
- Enantioselective synthesis of dihydrobenzofurans and dihydronaphthofurans via olefin isomerization/enantioselective intramolecular Alder-ene reaction of enynes catalyzed by Rh
- Preparation of axially chiral biaryl compounds by gold-catalyzed stereoselective intramolecular hydroarylation
- Preparation of chiral silylated homoallylic alcohols and diols by asymmetric addition of alcohols and aldehydes to silylbutadienes catalyzed by ruthenium complexes
- Preparation of chiral 3-alkyl-substituted indolines by tandem condensation-asymmetric hydrogenation of indoles with aldehydes, catalyzed by Bronsted acids and palladium BINAP complexes
- Rhodium-catalyzed asymmetric formal olefination or cycloaddition of 1,3-dicarbonyl compounds with 1,6-diynes or 1,6-enynes
Legal Information
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We present an article concerning BINAP/SEGPHOS® Ligands and Complexes.
Hydrogenation, Asymmetric Catalysis, Binap, SEGPHOS®, Aldol reaction, Alkenylation, Arylation, Mannich reaction, Fluorination, Michael addition, Hydrosilylation, Cycloaddition, Takasago
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service