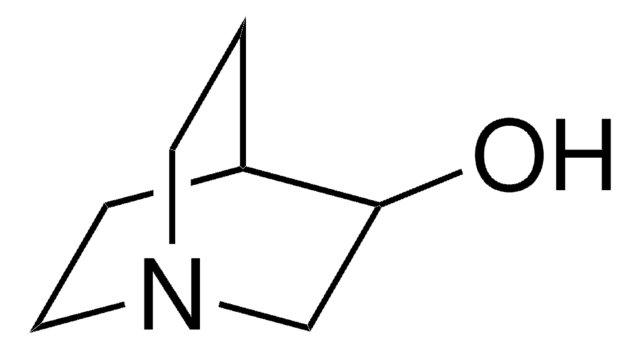
136824
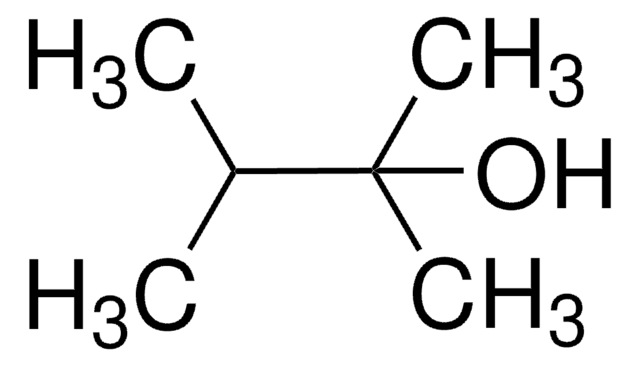
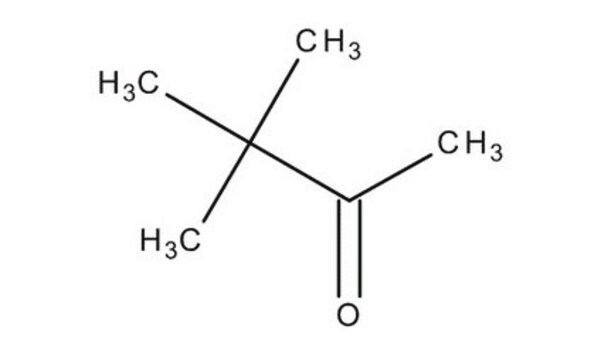
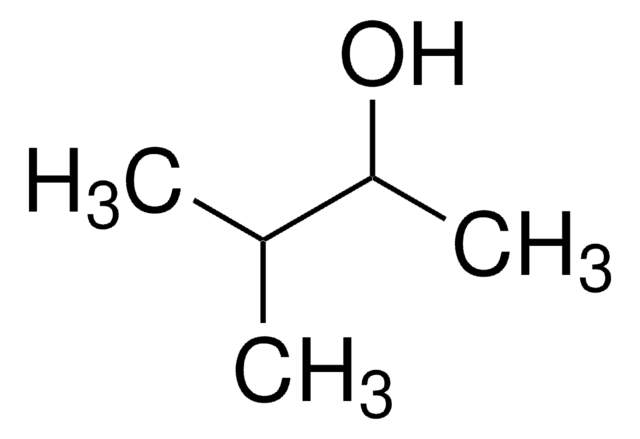
3,3-Dimethyl-2-butanol
98%
Synonym(s):
tert-Butyl methyl carbinol, Pinacoline alcohol, Pinacolyl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)3CCH(OH)CH3
CAS Number:
Molecular Weight:
102.17
Beilstein:
1718948
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.415 (lit.)
bp
119-121 °C (lit.)
mp
4.8 °C (lit.)
density
0.812 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
CC(O)C(C)(C)C
InChI
1S/C6H14O/c1-5(7)6(2,3)4/h5,7H,1-4H3
InChI key
DFOXKPDFWGNLJU-UHFFFAOYSA-N
Related Categories
General description
3,3-Dimethyl-2-butanol is a potential precursor for prohibited chemical weapons such as soman, a nerve agent. It is a synthetic analog of kairomone.
Application
3,3-Dimethyl-2-butanol (pinacolyl alcohol) can be used as a substrate:
- To study the oxidation of secondary alcohols to ketones using cyclic microwave heating technique.
- To prepare aryl ethers by reacting with aryl iodide using 4-pyrrolidinopyridine ligand via Cu-catalyzed Ullmann reaction.
3,3-Dimethyl-2-butanol was used in conversion of ribose- and glucose-binding proteins into receptors for pinacolyl methyl phosphonic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
78.8 °F - closed cup
Flash Point(C)
26 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chemical ionization mass spectral analysis of pinacolyl alcohol and development of derivatization method using p-tolyl isocyanate.
Murty MRVS, et al.
Analytical Methods : Advancing Methods and Applications, 2(10), 1599-1605 (2010)
Fast oxidation of secondary alcohols by the bromate-bromide system using cyclic microwave heating in acidic water
Paakkonen S, et al.
Tetrahedron Letters, 51(51), 6695-6699 (2010)
J T James et al.
Journal of applied toxicology : JAT, 7(5), 307-312 (1987-10-01)
Sprague-Dawley rats were given 15, 70 and 140 min exposures to 15 mg/l 3,3-dimethyl-2-butanol, pinacolyl alcohol (PA), or 6-hour exposures to 0.2, 1.0 and 5.0 mg/l PA (1 mg/l = 240 ppm). A 50% mortality rate was obtained at the
Ullmann CO coupling of sterically hindered secondary alcohols using excess amount of strongly coordinating monodentate ligands
Sugata H, et al.
Tetrahedron Letters, 58(10), 1015-1019 (2017)
W E Luttrell et al.
Biochemical pharmacology, 46(11), 2083-2092 (1993-12-03)
Soman (pinacolyl methylphosphonofluoridate), a highly toxic organophosphate compound, has been found to be a strong inhibitor of hepatic microsomal carboxylesterase in vitro, but an enhancer of carboxylesterase when administered in vivo. In response to this paradoxical observation, the objective of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service