129259
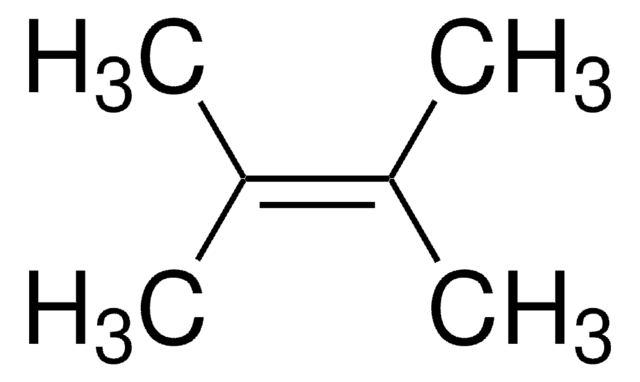
2,3-Dimethyl-2-butene
98%
Synonym(s):
Tetramethylethylene
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2C=C(CH3)2
CAS Number:
Molecular Weight:
84.16
Beilstein:
1361357
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
215 mmHg ( 37.7 °C)
Assay
98%
form
liquid
autoignition temp.
754 °F
refractive index
n20/D 1.412 (lit.)
bp
73 °C (lit.)
mp
−75 °C (lit.)
density
0.708 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C\C(C)=C(\C)C
InChI
1S/C6H12/c1-5(2)6(3)4/h1-4H3
InChI key
WGLLSSPDPJPLOR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,3-Dimethyl-2-butene undergoes hydrolysis to form hydroxyl radical.
Application
2,3-Dimethyl-2-butene was used in the preparation of thexyl NHC-borane (diMe-Imd-BH2thexyl).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
17.6 °F - closed cup
Flash Point(C)
-8 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xiangcheng Pan et al.
Journal of the American Chemical Society, 135(38), 14433-14437 (2013-08-29)
The hydroboration of alkenes of diverse structural types by assorted N-heterocyclic carbene boranes can be accomplished by addition of 5-10% diiodine. For example, reaction of 1,3-dimethylimidazol-2-ylidene borane (diMe-Imd-BH3) with 10% I2 followed by addition of 2,3-dimethyl-2-butene provided the corresponding thexyl
Maryline Pflieger et al.
Environmental science & technology, 47(12), 6239-6246 (2013-05-15)
In order to investigate the heterogeneous oxidation kinetics of the herbicide terbuthylazine (TERB), a stable and reproducible generation system of "dark" hydroxyl radical in the gas phase was developed and optimized using a PTR-MS. TERB was adsorbed on silica particles
L R Pohl et al.
Biochemical and biophysical research communications, 117(2), 367-371 (1983-12-16)
Although indirect evidence has suggested that liver microsomal cytochrome P-450 can reductively dehalogenate several compounds to carbene metabolites, there has been no direct proof for the formation of these reactive species. We report in this paper that carbenes can be
R Tolando et al.
Xenobiotica; the fate of foreign compounds in biological systems, 26(4), 425-435 (1996-04-01)
1. During anaerobic reductive incubation of liver microsomes, from either the pyridine- or phenobarbital-treated rat, with 1,1-dichloro-1-fluoroethane (HCFC-141b) in the presence of a NADPH-regenerating system, a time- and dose-dependent formation of reactive metabolites was detected as indicated by a depletion
Andrew T Lambe et al.
Environmental science & technology, 41(7), 2357-2363 (2007-04-19)
We present a novel method for continuous, stable OH radical production for use in smog chamber studies, especially those focused on organic aerosol aging. Our source produces OH radicals from the reaction of 2,3-dimethyl-2-butene and ozone and is unique as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service