P4234
Pyranose Oxidase from Coriolus sp.
recombinant, expressed in E. coli, ≥2.7 units/mg solid
Synonym(s):
Pyranose: Oxygen 2-Oxidoreductase
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
recombinant
expressed in E. coli
form
powder
specific activity
≥2.7 units/mg solid
shipped in
wet ice
storage temp.
−20°C
General description
Pyranose oxidase (P2O), a homotetrameric protein consists of a covalently bound flavin adenine dinucleotide (FAD). It is seen mostly among wood-degrading basidiomycetes.
Application
Pyranose Oxidase from Coriolus sp. has been used in the enzymatic oxidation of D-glucose (DG). It has also been used as a component in oxygen scavenging system (OSS) to increase the lifetime of the fluorophores.
Biochem/physiol Actions
Pyranose oxidase (P2O) can be used in clinical chemistry to determine 1,5-anhydro-d-glucitol marker, used for glycemic control in diabetes patients.
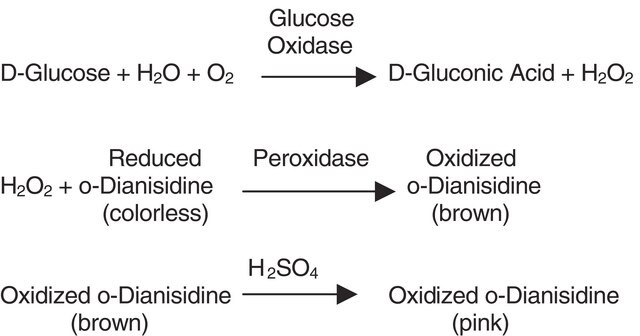
Pyranose oxidase (P2O) catalyzes the oxidation of aldopyranoses at position C-2 to yield the corresponding 2-ketoaldoses. The in vivo substrates of P2O are thought to be D-glucose, D-galactose, and D-xylose. They are oxidized to 2-keto-D-glucose (D-arabino-hexos-2-ulose, 2-dehydro-D-glucose), 2-keto-D-galactose (D-lyxo-hexos-2-ulose, 2-dehydro-D-galactose), and 2-keto-D-xylose (D-threopentos-2-ulose, 2-dehydro-D-xylose), respectively. Pyranose oxidase has significant activity with carbohydrates such as, L-sorbose, D-glucono-1,5-lactone, and D-allose. When pyranose oxidase catalyzes the oxidation of aldopyranoses, electrons are transferred to molecular oxygen which results in the formation of hydrogen peroxide.
Unit Definition
One unit produces 1.0 μmol of hydrogen peroxide per minute at 37 °C, pH 7.0.
Other Notes
Contains glutamate
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The influence of temperature on bioconversion of D-glucose into 2-keto-D-glucose by Pyranose oxidase
Ene MD, et al.
UPB Scientific Bulletin, Series B: Chemistry and Materials Science, 2 (2014)
Oliver Spadiut et al.
Microbial cell factories, 9, 14-14 (2010-03-11)
The heterologous production of the industrially relevant fungal enzyme pyranose 2-oxidase in the prokaryotic host E. coli was investigated using 3 different expression systems, i.e. the well-studied T7 RNA polymerase based pET21d+, the L-arabinose inducible pBAD and the pCOLD system.
Antti Lignell et al.
Nature communications, 8(1), 1830-1830 (2017-12-01)
The neural crest is an embryonic population of multipotent stem cells that form numerous defining features of vertebrates. Due to lack of reliable techniques to perform transcriptional profiling in intact tissues, it remains controversial whether the neural crest is a
Warintra Pitsawong et al.
The Journal of biological chemistry, 285(13), 9697-9705 (2010-01-22)
Pyranose 2-oxidase (P2O) catalyzes the oxidation by O(2) of d-glucose and several aldopyranoses to yield the 2-ketoaldoses and H(2)O(2). Based on crystal structures, in one rotamer conformation, the threonine hydroxyl of Thr(169) forms H-bonds to the flavin-N5/O4 locus, whereas, in
Tien-Chye Tan et al.
Journal of molecular biology, 402(3), 578-594 (2010-08-17)
Flavoenzymes perform a wide range of redox reactions in nature, and a subclass of flavoenzymes carry covalently bound cofactor. The enzyme-flavin bond helps to increase the flavin's redox potential to facilitate substrate oxidation in several oxidases. The formation of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service