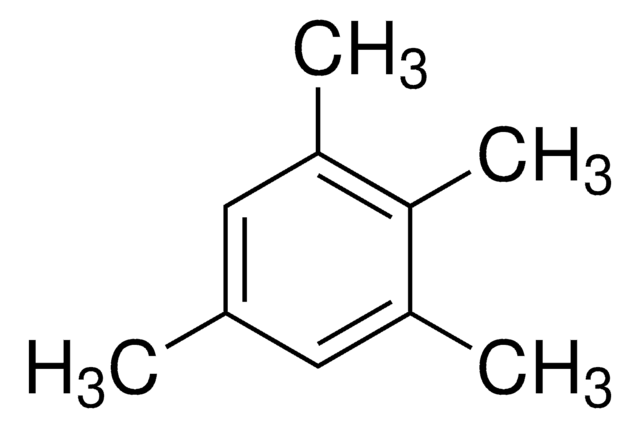
74658
1,2,4,5-Tetramethylbenzene
Standard for quantitative NMR, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
Synonym(s):
Durene, 1,2,4,5-Tetramethylbenzene
About This Item
Recommended Products
grade
Standard for quantitative NMR
certified reference material
TraceCERT®
Quality Level
vapor density
4.6 (vs air)
vapor pressure
160 mmHg ( 140 °C)
description
qNMR Standard for organic solvents (6.9 ppm / 2.2 ppm)
shelf life
limited shelf life, expiry date on the label
manufacturer/tradename
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
technique(s)
gas chromatography (GC): suitable
qNMR: suitable
mp
76-80 °C (lit.)
density
0.838 g/mL at 25 °C (lit.)
application(s)
cleaning products
cosmetics
environmental
flavors and fragrances
food and beverages
personal care
pharmaceutical
format
neat
SMILES string
Cc1cc(C)c(C)cc1C
InChI
1S/C10H14/c1-7-5-9(3)10(4)6-8(7)2/h5-6H,1-4H3
InChI key
SQNZJJAZBFDUTD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com.
Check out our entire range of quantitative NMR standards (qNMR standards)
Application
- OH-initiated Degradation of 1,2,4,5-Tetramethylbenzene: Research on the environmental behavior of 1,2,4,5-tetramethylbenzene highlights its degradation mechanisms and kinetics when exposed to OH radicals, underscoring its impact on air quality and potential ecological risks. This study is essential for environmental monitoring and management strategies involving aromatic hydrocarbons (Zhao et al., 2022).
- Low-Temperature Curing of Liquid Polybutadiene Using Nitrile Oxide: This study explores the application of 1,2,4,5-tetramethylbenzene in enhancing the curing process of liquid polybutadiene at low temperatures, illustrating its role as an effective solvent in the synthesis of polymers. Such applications are crucial for the development of advanced materials with improved performance characteristics (Li and Wang, 2022).
- Hygroscopic Properties of Calibration Standards for NMR Spectroscopy: 1,2,4,5-Tetramethylbenzene′s utility in nuclear magnetic resonance (NMR) spectroscopy as a calibration standard is detailed, focusing on its hygroscopic tendencies which affect the accuracy of quantitative analyses. This is vital for ensuring the reliability and precision of chemical measurements in research and industrial applications (Suiter and Widegren, 2021).
- Novel Sampling Strategy for Alive Animal Volatolome Extraction: The compound′s use in gas chromatography-mass spectrometry (GC-MS) based untargeted metabolomics for identifying pheromones in animal studies showcases its applicability in complex biological analyses, which is significant for advancements in veterinary and behavioral sciences (Lacalle-Bergeron et al., 2021).
- Microbial Degradation of Aromatic Hydrocarbons: The research on microbial degradation pathways of mobile aromatic hydrocarbons including 1,2,4,5-tetramethylbenzene provides insights into environmental remediation techniques. This is especially relevant for the biodegradation processes necessary for reducing pollution from dense non-aqueous phase liquids (DNAPLs) (Van Leeuwen et al., 2020).
Other Notes
Suitable NMR solvents: CDCl3
Recommended products
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Flam. Sol. 1
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 1
Flash Point(F)
165.2 °F - closed cup
Flash Point(C)
74 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The signal ratio of two different protons can be measured with tremendous precision, which enables the generation of certified reference materials for use as qNMR standards.
Protocols
-Xylene; Nonane; Propylbenzene; Mesitylene; 1,2,4-Trimethylbenzene; 1,2,3-Trimethylbenzene; 1,3-Diethylbenzene; 1,4-Dimethyl-2-ethylbenzene; 1,2-Dimethyl-4-ethylbenzene; Durene; 1,2,3,5-Tetramethylbenzene; 1,2,3,5-Tetramethylbenzene; 2-Methylnaphthalene (β)
GC Analysis of Hydrocarbons in Gasoline on Petrocol® DH, Isothermal
Related Content
ChemisTwin is your new online digital assistant for NMR result interpretation. It will save you time by automating the interpretation of routine NMR analysis for structure confirmation as well as quantitative NMR (qNMR) measurements using qNMR eRMs from our verified electronic Reference Materials database.
ChemisTwin is your new online digital assistant for NMR result interpretation. It will save you time by automating the interpretation of routine NMR analysis for structure confirmation as well as quantitative NMR (qNMR) measurements using qNMR eRMs from our verified electronic Reference Materials database.
ChemisTwin is your new online digital assistant for NMR result interpretation. It will save you time by automating the interpretation of routine NMR analysis for structure confirmation as well as quantitative NMR (qNMR) measurements using qNMR eRMs from our verified electronic Reference Materials database.
ChemisTwin is your new online digital assistant for NMR result interpretation. It will save you time by automating the interpretation of routine NMR analysis for structure confirmation as well as quantitative NMR (qNMR) measurements using qNMR eRMs from our verified electronic Reference Materials database.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate Selectophore™](/deepweb/assets/sigmaaldrich/product/structures/251/439/7a621e74-bfd1-4a43-833c-09adfcc1e0b3/640/7a621e74-bfd1-4a43-833c-09adfcc1e0b3.png)