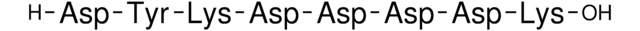Recommended Products
form
liquid
mol wt
~49 kDa
shipped in
dry ice
storage temp.
−20°C
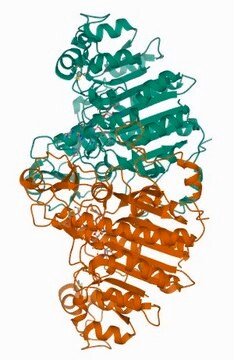
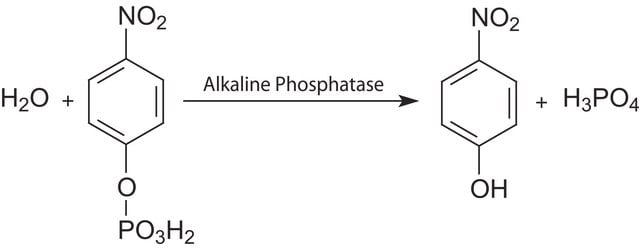
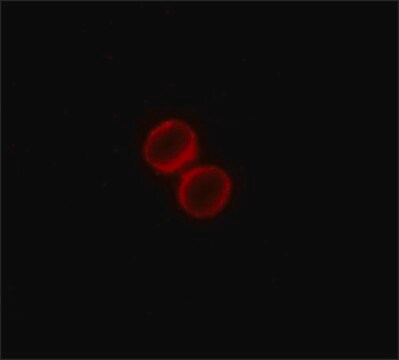
General description
Application
Biochem/physiol Actions
Other Notes
Physical form
Preparation Note
Legal Information
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Comparison of elution techniques for small-scale protein purification of FLAG® tag proteins using anti-FLAG® M2 magnetic beads.
Protocols
Protocol for immunoprecipitation (IP) of FLAG fusion proteins using M2 monoclonal antibody 4% agarose affinity gels
Related Content
Protein purification techniques, reagents, and protocols for purifying recombinant proteins using methods including, ion-exchange, size-exclusion, and protein affinity chromatography.
Protein expression technologies for expressing recombinant proteins in E. coli, insect, yeast, and mammalian expression systems for fundamental research and the support of therapeutics and vaccine production.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service